Lomitapide , 98%(HPLC) , 182431-12-5
Synonym(s):
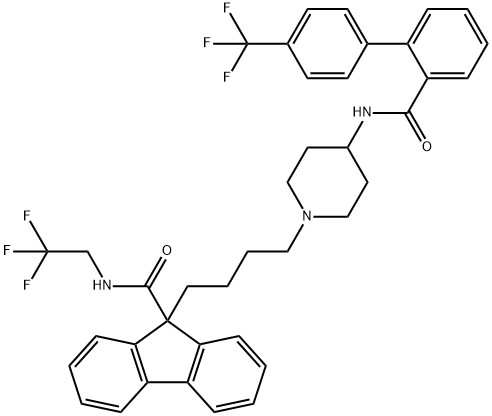
AEGR-733;BMS-201038;N-(2,2,2-Trifluoroethyl)-9-[4-[4-[[[4′-(trifluoromethyl)[1,1′-biphenyl]2-yl]carbonyl]amino]-1-piperidinyl]butyl]9H-fluoren-9-carboxamde
CAS NO.:182431-12-5
Empirical Formula: C39H37F6N3O2
Molecular Weight: 693.72
MDL number: MFCD16620494
| Pack Size | Price | Stock | Quantity |
| 25MG | RMB135.20 | In Stock |
|
| 50mg | RMB239.20 | In Stock |
|
| 250mg | RMB799.20 | In Stock |
|
| 1g | RMB2399.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 142°C(lit.) |
| Boiling point: | 778.2±60.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 12.66±0.20(Predicted) |
| form | powder |
| color | white to beige |
Description and Uses
Lomitapide was approved by the US FDA in December 2012 for the treatment of patients with familial hypercholesteremia (referred to as HoFH) in conjunction with a low-fat diet andother lipid-lowering treatments. Lomitapide was discovered from a high-through put screen that identified several structurally distinct MTP inhibitors. Combination of key structural features from two structurally distinct HTS hits provided potent MTP inhibitors. Parallel analog synthesis led to lomitapide as an optimized structure. Lomitapide was synthesized via alkylation of 9-fluorenylcarboxylic acid with 1,4-dibromobutane which, after trifluoroethylamide formation, provided a bromide intermediate that was displaced by Boc-4-aminopiperidine. Introduction of the 4'-trifluoromethylbiphenylcarboxamide gave lomitapide, which was found to inhibit MTP with an IC50 of 0.5 nM and to exhibit good cholesterol-lowering efficacy in Sprague–Dawley rats (intravenous and oral ED50~0.2 mg/kg).
Lomitapide has been used as a microsomal triglyceride transfer protein (MTP) inhibitor to study its effects on very-low-density lipoproteins (VLDL) export in mouse hepatocytes.
Safety
| HS Code | 2933.39.4100 |
| Hazardous Substances Data | 182431-12-5(Hazardous Substances Data) |