Apremilast , ≥99% , 608141-41-9
CAS NO.:608141-41-9
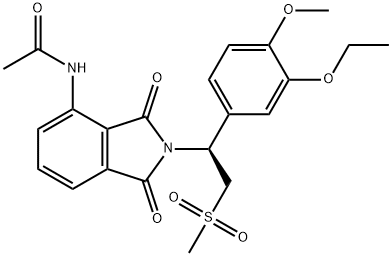
Empirical Formula: C22H24N2O7S
Molecular Weight: 460.5
MDL number: MFCD18782607
EINECS: 807-237-6
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB23.20 | In Stock |
|
| 25MG | RMB71.20 | In Stock |
|
| 100MG | RMB183.20 | In Stock |
|
| 250mg | RMB319.20 | In Stock |
|
| 1g | RMB799.20 | In Stock |
|
| 5g | RMB2399.20 | In Stock |
|
| 25g | RMB7999.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 152-156°C |
| Boiling point: | 741.3±60.0 °C(Predicted) |
| Density | 1.381 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated) |
| pka | 14.01±0.20(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| InChIKey | IMOZEMNVLZVGJZ-QGZVFWFLSA-N |
| SMILES | C(NC1=CC=CC2=C1C(=O)N([C@@H](C1=CC=C(OC)C(OCC)=C1)CS(C)(=O)=O)C2=O)(=O)C |
| CAS DataBase Reference | 608141-41-9 |
Description and Uses
Apremilast is the first and only oral phosphodiesterase IV (PDE- 4) inhibitor and anti-tumor necrosis factor alpha (TNFa) agent launched in the USA by Celgene for the treatment of active psoriatic arthritis (PsA). Apremilast was also approved for the treatment of moderate to severe plaque psoriasis in the USA. Later, this drug was also approved in the European Union (EU) for both indications. Although multiple approaches to the synthesis of apremilast have been described, the most likely process scale approach involves the construction of the challenging stereogenic benzylic carbon center by catalytic asymmetric hydrogenation.
Apremilast is an oral phosphodiesterase 4 inhibitor used in the treatment of adults with active psoriatic arthritis and adults with moderate to severe plaque psoriasis.
Safety
| Symbol(GHS) |   GHS08,GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H361 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P264-P270-P301+P312-P330-P501 |
| Hazardous Substances Data | 608141-41-9(Hazardous Substances Data) |