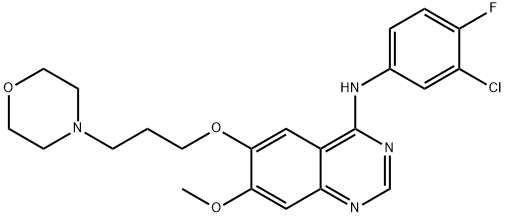
Gefitinib (ZD1839) , ≥99% , 184475-35-2
Synonym(s):
N-(3-Chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine;ZD1839
CAS NO.:184475-35-2
Empirical Formula: C22H24ClFN4O3
Molecular Weight: 446.9
MDL number: MFCD04307832
EINECS: 643-034-7
| Pack Size | Price | Stock | Quantity |
| 50MG | RMB23.20 | In Stock |
|
| 250MG | RMB31.20 | In Stock |
|
| 1G | RMB48.80 | In Stock |
|
| 5G | RMB143.20 | In Stock |
|
| 25G | RMB553.60 | In Stock |
|
| 100G | RMB1998.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 119-1200C |
| Boiling point: | 586.8±50.0 °C(Predicted) |
| Density | 1.322±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble in DMSO (up to 40 mg/ml) or in Ethanol (up to 4 mg/ml). |
| pka | 7.00±0.10(Predicted) |
| form | powder |
| color | white to beige |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 184475-35-2(CAS DataBase Reference) |
Description and Uses
Gefitinib was introduced in Japan as a daily oral monotherapy for the treatment of inoperable or recurrent non-small cell lung cancers (NSCLC). This anilinoquinazoline derivative can be synthesized in 6 steps starting from 6,7-dimethoxyquinazolin-4(3H)-one by successive monodemethylationlacetylation of the 6-hydroxy-group followed by chlorination and reaction with 3-chloro-4-fluoroaniline, finally deacetylation and alkylation with 3-(4-morpholinyl)propylbromide complete the synthesis. Gefitinib reversibly inhibits the activity of the epidermal growth factor receptor tyrosine kinase (EGRF TK). This inhibits autophosphorylation of EGRF and blocks the cascade of intracellular events which have been implicated in the proliferation, survival and metastasis of cancer cells. Gefitinib diplays good selectivity for the EGRF TK relative to other growth factors in human umbilical endothelial cells. It is similarly selective relative to other kinases, for example cerB2. Data from two large phase II studies in patients with pretreated NSCLC have shown that gefitinib induces a response rate approaching 20% in patients receiving the agent as a second line therapy and approximately 10% in those pretreated with more lines of chemotherapy. Gefitinib has good bioavailability and is metabolized in the liver via the cytochrome P450 3A4 enzyme system with a mean elimination half life of 28 h. Gefitinib has been generally well tolerated in cancer patients with predominant side effects being acne-like skin-rash, diarrhea, nausea, vomiting and mild to moderate myelosuppression. .
Gefitinib (Iressa, ZD-1839) is an EGFR inhibitor for Tyr1173, Tyr992, Tyr1173 and Tyr992 in the NR6wtEGFR and NR6W cells with IC50 of 37 nM, 37nM, 26 nM and 57 nM, respectively.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P301+P312+P330-P305+P351+P338 |
| Safety Statements | 24/25 |
| HS Code | 29349990 |