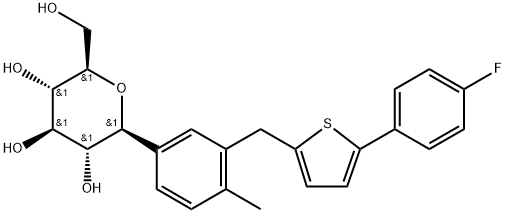
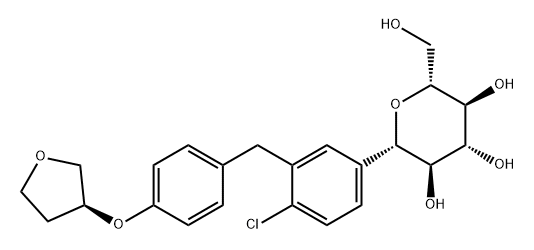
Canagliflozin , ≥98% , 842133-18-0
CAS NO.:842133-18-0
Empirical Formula: C24H25FO5S
Molecular Weight: 444.52
MDL number: MFCD18251436
EINECS: 695-192-1
| Pack Size | Price | Stock | Quantity |
| 10MG | RMB79.20 | In Stock |
|
| 50MG | RMB138.40 | In Stock |
|
| 250MG | RMB426.40 | In Stock |
|
| 1g | RMB1308.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 68 - 72°C |
| Boiling point: | 642.9±55.0 °C(Predicted) |
| Density | 1.364 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (40 mg/ml) |
| form | solid |
| pka | 13.34±0.70(Predicted) |
| color | White |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| InChIKey | XTNGUQKDFGDXSJ-XDLHWMRXNA-N |
| SMILES | O[C@@H]1[C@H]([C@H](O)[C@@H](CO)O[C@H]1C1C=CC(C)=C(CC2=CC=C(C3C=CC(F)=CC=3)S2)C=1)O |&1:1,2,3,5,9,r| |
| CAS DataBase Reference | 842133-18-0 |
Description and Uses
In March 2013, the US FDA approved canagliflozin (JNJ-28431754; TA-7284) for the treatment of type 2 diabetes in adults. Canagliflozin is the first US-approved sodium-glucose co-transporter (SGLT) inhibitor for the treatment of type 2 diabetes. Inhibition of renal SGLT suppresses glucose reabsorption, which permits glucose excretion into urine and reduction of hyperglycemia. Canagliflozin was discovered through structural modifications of phlorizin, a known inhibitor of renal glucose reabsorption. Early modifications of OH groups on the glucose moiety were insufficient to adequately impair hydrolysis by intestinal β-glucosidase. Introduction of the C-glucoside moiety, as in the clinical candidate dapagliflozin, afforded sufficient resistance to hydrolysis. Finally, incorporation of the thiophene moiety in canagliflozin provided improved potency for hSGLT2 (exclusive to kidney), IC50=2.2 nM, while offering significant selectivity over hSGLT1 (in kidney and heart), IC50=910 nM. Hyperglycemic, high-fat (HF) diet fedmice (KKstrain) that received a single 3 mg/kg oral dose of canagliflozin had a 48% reduction in blood glucose levels after 6 h versus vehicle-treatedmice. Noteworthy in themultistep synthesis of canagliflozin is the stereoselective formation of the β-C-glucoside which is accomplished by coupling of the aryllithiumaglycone with 2,3,4,6-tetra-O-trimethylsilyl-β-Dgluconolactone followed by desilylation and stereoselective reduction with triethylsilane and boron trifluoride etherate.
Canagliflozin is a sodium/glucose cotransporter 2 (SGLT2) inhibitor. Canagliflozin has been shown to dose dependently reduce calculated renal threshold for glucose excretion and increase urinary glucose excretion. Canagliflozin is a candidate for the treatment of type 2 diabetes and obesity.
Safety
| Symbol(GHS) |   GHS08,GHS05 |
| Signal word | Danger |
| Hazard statements | H361-H318 |
| Precautionary statements | P280-P305+P351+P338-P310-P201-P202-P281-P308+P313-P405-P501 |
| Hazardous Substances Data | 842133-18-0(Hazardous Substances Data) |