Atomoxetine HCl , ≥98% , 82248-59-7
Synonym(s):
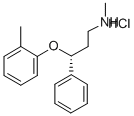
(R)-N-Methyl-γ-(2-methyl-phenoxy)benzenepropanamine hydrochloride;(R)-Tomoxetine hydrochloride;Atomoxetine hydrochloride
CAS NO.:82248-59-7
Empirical Formula: C17H22ClNO
Molecular Weight: 291.82
MDL number: MFCD06410992
EINECS: 629-925-3
| Pack Size | Price | Stock | Quantity |
| 1G | RMB120.80 | In Stock |
|
| 50MG | RMB151.20 | In Stock |
|
| 250MG | RMB186.40 | In Stock |
|
| 5G | RMB464.80 | In Stock |
|
| 25g | RMB1932.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 167-169°C |
| alpha | D25 -38.01°; 36525 -177.26° (c = 1 in methanol); D23 -41.37° (c = 1.02 in methanol); D25 -40.3° (c = 0.94 in ethanol) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | soluble in Methanol |
| form | solid |
| pka | 10.13(at 25℃) |
| color | White to Almost white |
| optical activity | -37.10°(C=0.01g/mL, MEOH, 589nm) |
| Water Solubility | Soluble to 50 mM in water with gentle warming |
| Merck | 14,863 |
| BCS Class | 1? |
| InChI | InChI=1/C17H21NO.ClH/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15;/h3-11,17-18H,12-13H2,1-2H3;1H/t17-;/s3 |
| InChIKey | LUCXVPAZUDVVBT-UNTBIKODSA-N |
| SMILES | O([C@H](CCNC)C1C=CC=CC=1)C1=CC=CC=C1C.Cl |&1:1,r| |
| CAS DataBase Reference | 82248-59-7(CAS DataBase Reference) |
Description and Uses
Atomoxetine is the first non-stimulant marketed for the treatment of attention deficit hyperactivity disorder (ADHD). It is the R-stereoisomer of the racemate tomoxetine and is a selective and potent norepinephrine uptake inhibitor (Ki=0.7–1.9 nM) that is devoid of binding to monoamine receptor. It also has little effect on dopamine and serotonin reuptake or acetylcholine, H1 histamine, alpha1 or alpha1-adrenergic or dopamine receptors. It is prepared from racemic 1-phenylbut-3-en-1-ol via a selective enzymatic acylation leaving the desired S-stereoisomer as the alcohol. This alcohol is converted via a Mitsunobu reaction with ortho-cresol to the corresponding ether with isomeric R-configuration. Ozonolysis and reduction steps provided the terminal alcohol that is mesylated and displaced with methylamine. Its selectivity for norepinephrine relative to dopamine inhibition was demonstrated in vivo preclinically. In a two-lever (two condition) discriminative stimulus effect study in squirrel monkeys, tomoxetine and other norepinephrine uptake inhibitors substituted for cocaine under low-dose training conditions, whereas dopamine uptake inhibitors substituted for cocaine in both low and high-dose conditions. In clinical ADHD studies in adolescents, it was significantly different from placebo in 1.2 and 1.8 mpk/day dosing. In the clinical study in adults using the CAARS scale a 95 mg/day dose provided greater than 30% improvement in total scores. Atomoxetine is about 63% orally bioavailable, is highly protein bound (98%, primarily to albumin) and has a half-life of about 5.2 h. It is metabolized by CYP2D6 resulting in differential clearance for poor metabolizers (halflife of 19 h with a 10 times higher AUC) relative to extensive metabolizers. The total daily dose for children, adolescents and adults is a maximum of 100 mg/day. Common side effects in children and adults include nausea, decreased appetite, and dizziness. Adults may also have insomnia.
(R)-Tomoxetine hydrochloride has been used as a noradrenaline reuptake inhibitor:
- to study the role of L-threo-3,4-dihydroxyphenylserine (L-DOPS) in the pathogenesis of Alzheimer′s disease in mice
- to study its effects on set shifting in rats
- to study its effects on rat brain as a result of its long-term use
Safety
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310-P311 |
| Hazard Codes | F,T |
| Risk Statements | 11-23/24/25-39/23/24/25 |
| Safety Statements | 22-24/25-45-36/37-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| HS Code | 29221990 |