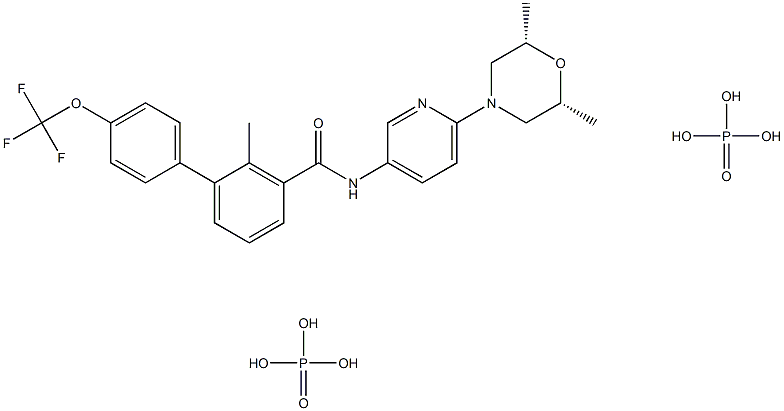
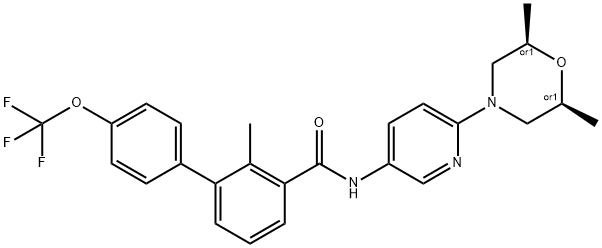
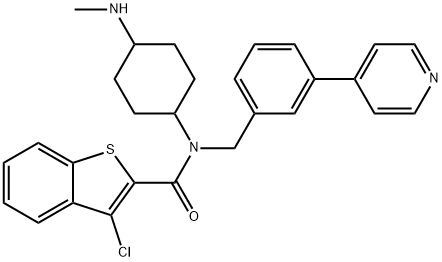
Erismodegibdiphosphate , ≥99% , 1218778-77-8
| Pack Size | Price | Stock | Quantity |
| 5mg | RMB848.00 | In Stock |
|
| 10mg | RMB1017.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| storage temp. | Store at -20°C |
| solubility | ≥27.85 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| form | White solid. |
| color | White to off-white |
Description and Uses
Sonidegib phosphate (XXIX), an orally bioavailable, small molecule smoothened (SMO) receptor antagonist developed by Novartis, was approved in 2015 in Switzerland, the U.S., and the EU for the treatment of adult patients with advanced or locally advanced basal cell carcinoma (BCC). BCC is the most frequently diagnosed skin cancer, constituting 80% of all nonmelanoma skin cancers. While most BCCs can be treated through surgery or radiation therapy, some patients (<10%) have more advanced tumors for which surgery may be contraindicated or impractical; treatment options for these patients are limited. SMO, a G-protein coupled receptor-like protein (GPCR), is a key regulator in the hedgehog (Hh) pathway, which is activated in a number of tumors including BCC. In a multicenter clinical trial for sonidegib, an objective response rate of 43% was observed for patients with locally advanced BCC (dosing at 200 mg once daily), with sustained clinically meaningful responses based on an 18-month analysis. Sonidegib joins vismodegib as marketable treatments of BCC. Vismodegib, which was approved in 2012 as a first-in-class SMO receptor antagonist, represented the first Hedgehog signaling pathway targeting agent to gain FDA approval.
NVP-LDE225 Diphosphate Salt is a potent, selective, and orally bioavailable Smoothened (Hedgehog Signaling Pathway) antagonist.