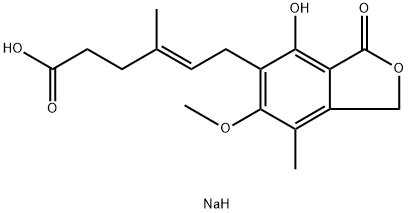
4-Hexenoicacid,6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-,sodiumsalt(1:1),(4E)- , 95% , 37415-62-6
| Pack Size | Price | Stock | Quantity |
| 100mg | RMB640.00 | In Stock |
|
| 1g | RMB2080.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| storage temp. | 2-8°C |
| form | Solid |
| color | White to off-white |
Description and Uses
Mycophenolate sodium, an immunosuppressive agent, was launched in Switzerland as an oral treatment in combination with Neoral? and corticosteroids for the prevention of acute transplant rejection in adult patients receiving allogeneic renal transplantation. In contrast to the previously marketed product mycophenolate mofetil (MMF, CellCept?), which is a prodrug and must be converted to mycophenolic acid (MPA) in vivo, Myfortic? contains MPA itself as the active ingredient. Myfortic? was designed to enhance the therapeutic efficacy of MPA through increased tolerability relative to systemic exposure. Unlike MMF, which is absorbed in the stomach, the enteric-coated formulation of MPA sodium is mainly absorbed in the small intestine, thus protecting the upper GI tract from the side effects of MPA. MPA is an inhibitor of inosine monophosphate dehydrogenase (IMDPH), a vital enzyme in the de novo pathway of purine biosynthesis. Proliferating lymphocytes rely principally on this pathway for purine production,thus rendering them succeptible to depletion of purine bases by MPA. Inhibition of IMPDH by MPA results in the inhibition of both T- and B-lymphocyte proliferation upon antigen challenge and facilitates the prevention of acute graft rejection. Following oral administration of MPA sodium, the tmax of MPA is 1.5–2 h, with a mean absolute bioavailability of 71% and a mean half-life of 11.7 h. MPA is primarily metabolized in the liver by glucuronidation and excreted mainly in the urine as the metabolite. The recommended dosage regimen of MPA sodium is 720 mg twice daily, which provides equimolar amounts of MPA compared with MMF 1000 mg twice daily. In two major clinical trials in 748 patients, Myfortic? was demonstrated to be a highly potent and well-tolerated immunosuppressant for new renal transplant patients. A trend was seen towards fewer dose reductions due to GI intolerability and less serious infections relative to other MPA drugs. The most common adverse events associated with MPA treatment are diarrhea and leukopenia.
Mycophenolic Acid Monosodium Salt in its enteric-coated form is used as an immunosuppressive agent in organ transplantation and autoimmune diseases.Environmental contaminants; Food contaminants
Safety
| Symbol(GHS) |   GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | H413-H400-H302-H360 |
| Precautionary statements | P273-P391-P501-P264-P270-P301+P312-P330-P501 |
| HS Code | 2932202000 |
| Toxicity | LD50 in mice (mg/kg): 1176±151 orally; 568±53 i.p. (Williams) |