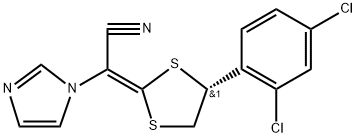
Luliconazole , 10mMinDMSO , 187164-19-8
CAS NO.:187164-19-8
Empirical Formula: C14H9Cl2N3S2
Molecular Weight: 354.27
MDL number: MFCD00953915
EINECS: 247-960-9
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB239.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 152 °C |
| Boiling point: | 499.1±55.0 °C(Predicted) |
| Density | 1.52±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| pka | 3.76±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 187164-19-8 |
Description and Uses
Luliconazole is a member of the imidazole class of antifungal agents, with specific utility as a dermatological antimycotic drug. It was launched in Japan as a topical agent for the treatment of athlete’s foot. Luliconazole is an optically active drug with (R)-configuration at its chiral center. It is structurally related to lanoconazole, which has been marketed as a racemic mixture since 1994. As with other azole antifungal drugs, the mechanism of action of luliconazole is the inhibition of sterol 14-a-demethylase, and subsequently, inhibition of ergosterol biosynthesis. The in vivo activity of luliconazole against dermatophytes has been evaluated in the guinea pig model of tinea pedis. In this study, a 1% topical solution of luliconazole, administered once daily for seven days, achieved complete mycologic cure. Additionally, there were no occurrences of relapse for up to 16 weeks after the treatment. No data is currently available on the clinical efficacy of luliconazole. The chemical synthesis of luliconazole involves the condensation of 1-(cyanomethyl)imidazole with carbon disulfide to produce a dithioate intermediate, and subsequent alkylation with either the mesylate derivative of (S′)-1-(2,4-dichlorophenyl)-2-bromoethanol or the bis-mesylate derivative of (S′)-1-(2,4-dichlorophenyl)ethane-1,2-diol. .
Luliconazole is an azole antifungal drug. It was approved by the FDA (USA) in November 2013 and is marketed under the brand name Luzu. Luliconazole is also approved in Japan.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H319-H315 |
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P264-P270-P301+P312-P330-P501 |
| HS Code | 2933.29.2000 |