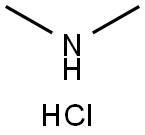
Dimethylaminehydrochloride , 10mMinDMSO , 506-59-2
Synonym(s):
Dimethylammonium chloride
CAS NO.:506-59-2
Empirical Formula: C2H8ClN
Molecular Weight: 81.54
MDL number: MFCD00012477
EINECS: 208-046-5
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 170-173 °C(lit.) |
| Boiling point: | 95.79°C (rough estimate) |
| Density | 0.8949 (rough estimate) |
| bulk density | 500kg/m3 |
| vapor pressure | <0.1 hPa (25 °C) |
| refractive index | 1.4202 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble0.5g/10 mL |
| form | Crystalline Powder or Crystals |
| color | White to off-white |
| PH | 5 (100g/l, H2O, 20℃) |
| Water Solubility | 3000 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,3228 |
| BRN | 3589311 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | IQDGSYLLQPDQDV-UHFFFAOYSA-N |
| LogP | -3.28 |
| CAS DataBase Reference | 506-59-2(CAS DataBase Reference) |
| EPA Substance Registry System | Methanamine, N-methyl-, hydrochloride (506-59-2) |
Description and Uses
Dimethylamine hydrochloride is the raw material of organic synthesis, the catalyzer of analysis as an acetylize, and the preparation of azoviolet and dimethylamine aqueous solution. Dimethylamine hydrochloride deoxidation is very capable, and corrodibility is vital, so be usually used in cleaning-type soldering flux. Preparation technology is for slowly splashing into hydrochloric acid in dimethylamine for tradition, and the speed that splashes into is no more than 15 ℃ with temperature, and pH=7-8 is limited. After the reaction finishes, activated carbon decolorizing is added, the filtrate is adjusted to pH=3-4 with hydrochloric acid, decompression is steamed water to the greatest extent, obtaining dimethylamine hydrochloride.
Dimethylamine hydrochloride is used as an intermediate in the manufacture of pharmaceuticals like ranitidine and metformin, tramadol and amlodipine. It is used as a precursor of atrazine. It is associated with sodium acetate and used to carry out the Willgerodt- Kindler reaction to prepare amides. Its free base reacts with carbon disulfide to get dimethyldithiocarbamate which is used in rubber vulcanization. It is involved in the synthesis of dimethyl-(1-methyl-pyrrol-2-ylmethyl)-amine by reacting with 1-methyl pyrrole and formaldehyde.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319 |
| Precautionary statements | P264-P270-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36/37-37/39-36 |
| WGK Germany | 1 |
| RTECS | IQ0220000 |
| F | 3-9 |
| TSCA | Yes |
| HS Code | 29211190 |
| Toxicity | LD50 in mice (g/kg): 1.21 i.v., 2.00 s.c. (Hazard); LC50 in mice (ppm): 7650 (48 hr), 4725 (14 day); in rats (ppm): 4540 (6 hr) (Steinhagen) |