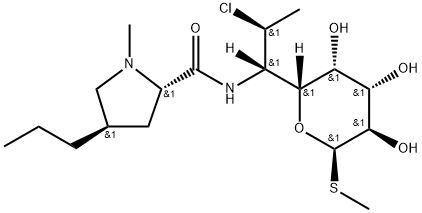
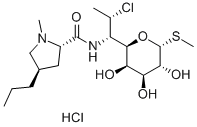
Clindamycin , 10mMinDMSO , 18323-44-9
CAS NO.:18323-44-9
Empirical Formula: C18H33ClN2O5S
Molecular Weight: 424.98
MDL number: MFCD00072005
EINECS: 242-209-1
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB559.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| alpha | D +214° (chloroform) |
| Boiling point: | 134°C (rough estimate) |
| Density | 1.1184 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO:52.5(Max Conc. mg/mL);123.54(Max Conc. mM) DMF:30.0(Max Conc. mg/mL);70.59(Max Conc. mM) Ethanol:52.5(Max Conc. mg/mL);123.54(Max Conc. mM) PBS (pH 7.2):0.2(Max Conc. mg/mL);0.47(Max Conc. mM) |
| form | A crystalline solid |
| pka | 7.6(at 25℃) |
| color | White to light yellow |
| BCS Class | 1 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 18323-44-9(CAS DataBase Reference) |
| EPA Substance Registry System | L-threo-?-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio- (18323-44-9) |
Description and Uses
Clindamycin, a derivative of lincomycin, was first isolated from Streptomyces lincolnesis in 1962 and became commercially available in 1966. The name originates from Lincoln, Nebraska, where the organism was first isolated from a soil sample. The parent compound of clindamycin is lincomycin, which is produced by actinomycete, Streptomyces liconelnensis, which belongs to the order of Actinobacteria. It replaced lincomycin use because of its better absorption and clinical spectrum. Lincomycin belongs to the lincosamides, which is a class of antibiotics. The compounds use as antibiotic agents stems from their ability to interfere with the protein synthesis of bacteria. The chemical modification of lincomycin, clindamycin, has proven to be superior to the natural product for clinical applications. Clindamycin is better absorbed from the gastrointestinal tract and food does not interfere with its absorption. It is also eight times more active against aerobic gram-positive cocci, and it has proven activity against gram-positive and gram-negative anaerobic bacteria as well as protozoal organisms like Toxoplasma and Plasmodium species.
Clindamycin is a semi-synthetic analogue of lincomycin, prepared by chloride substitution of the exocyclic sugar hydroxy group. This affords a more hydrophobic compound with improved pharmacodynamics. Like other members of the lincosamide family, clindamycin is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Clindamycin acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Clindamycin has been extensively studied with over 8,000 literature citations.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H362-H319 |
| Precautionary statements | P201-P260-P263-P264-P270-P308+P313-P264-P280-P305+P351+P338-P337+P313P |
| Hazardous Substances Data | 18323-44-9(Hazardous Substances Data) |
| Toxicity | LD50 subcutaneous in rat: 2618mg/kg |