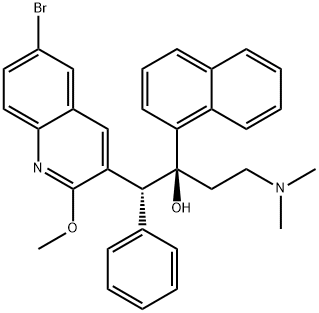
bedaquiline , 10mMinDMSO , 843663-66-1
CAS NO.:843663-66-1
Empirical Formula: C32H31BrN2O2
Molecular Weight: 555.5
MDL number:
EINECS: 1308068-626-2
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB314.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 104 °C |
| Boiling point: | 702.7±60.0 °C(Predicted) |
| Density | 1.322±0.06 g/cm3(Predicted) |
| storage temp. | Room temperature |
| solubility | Soluble in DMSO (10 mg/ml) |
| form | solid |
| pka | 13.05±0.29(Predicted) |
| color | White |
| optical activity | [α]/D 195.0 to 155.0° (c = 0.5g/100mL in DMF) |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 843663-66-1 |
Description and Uses
In December 2012, the US FDA approved bedaquiline as part of combination therapy for the treatment of multi-drug resistant tuberculosis (MDRTB). Bedaquiline is the first drug approved for MDR-TB and is the first approval from a new class of antituberculosis agents in the past 40 years. Due to the high unmetmedical need for treating MDR-TB, the FDA granted bedaquiline accelerated approval based on Phase II results, providing patients access to the drug while additional clinical studies are carried out. Bedaquiline (also known as TMC207 and R207910) is a diarylquinoline that was discovered from a high-throughput, whole-cell screening strategy with Mycobacterium smegmatis used as a surrogate for M. tuberculosis. Bedaquiline is a single enantiomer of an initial screening hit. Bedaquiline has potent and selective activity against mycobacteria, and is active against both drug-sensitive and drug-resistant M. tuberculosis. The mechanism of action of bedaquiline is unique amongst anti-TB drugs and involves inhibition of mycobacterial ATP synthase; it is not active against human ATP synthase. Bedaquiline has in vivo activity in numerous preclinical models of TB infection, alone and in combination with other anti-TB agents, and has bactericidal activity in established TB infection models. Bedaquiline is synthesized in five steps from 3-phenylpropionic acid and para-bromoaniline. Following amide formation, treatment with POCl3 and DMF under Vilsmeier–Hack conditions gave a 2-chloroquinoline product. Treatment with sodium methoxide, followed by condensation with 3-(dimethylamino)-1-(naphthalen-1-yl)propan-1-one, and separation of isomers gave bedaquiline.
Labeled Bedaquiline, intended for use as an internal standard for the quantification of Bedaquiline by GC- or LC-mass spectrometry.
Safety
| Symbol(GHS) |    GHS08,GHS09,GHS06 |
| Signal word | Danger |
| Hazard statements | H400-H373-H301-H410 |
| Precautionary statements | P273-P391-P501-P264-P270-P301+P310-P321-P330-P405-P501-P273-P391-P501-P260-P314-P501 |
| HS Code | 2933499090 |
| Hazardous Substances Data | 843663-66-1(Hazardous Substances Data) |