Perampanel , ≥98% , 380917-97-5
CAS NO.:380917-97-5
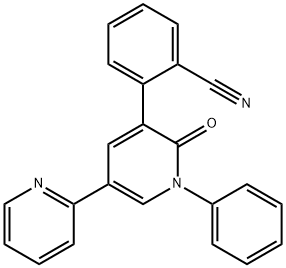
Empirical Formula: C23H15N3O
Molecular Weight: 349.38
MDL number: MFCD19443693
EINECS: 691-969-4
PRODUCT Properties
| Boiling point: | 619.1±55.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 4.73±0.19(Predicted) |
Description and Uses
In October 2012, the US FDA approved perampanel for the treatment of partial onset seizures in epileptic patients who are at least 12 years old. Perampanel is the first AMPA receptor antagonist to receive FDA approval as an AED. AMPA glutamate receptors are found primarily on postsynaptic neurons in the brain. As a selective, noncompetitive antagonist of AMPA, parampanel prevents ion channel opening and reduces propagation of action potential. Parampanel was discovered through lead optimization of a commercially available compound, 2,4-diphenyl-4H-[1,3,4]oxadiazin-5-one, which was identified by high-throughput screening of a compound collection employing a rat cortical neuron AMPA-induced cell-death assay. Modifications of aromatic rings at positions 1, 3, and 5 while changing the core to pyridone led to parampanel which inhibited AMPA-induced calcium influx (IC50=60 nM). Parampanel had a minimum effective oral dose of 2 mg/kg in an AMPA-induced mouse seizure model. The synthesis of parampanel was accomplished via a 6-step route utilizing Suzuki–Miyaura couplings and modified Ullmann reactions for incorporation of aryl groups.
Isotope labelled Perampanel (P285520), is an antiepileptic drug. It inhibits α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-induced increases in intracellular Ca2+ and selectively blocks AMPA receptor-mediated synaptic transmission, thus reducing neuronal excitation.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H413-H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |