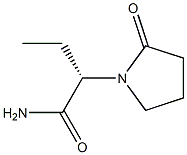
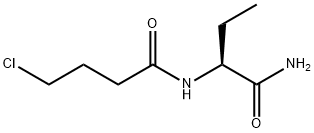
Levetiracetam , ≥99% , 102767-28-2
Synonym(s):
(αS)-α-Ethyl-2-oxo-1-pyrrolidineacetamide;2(S)-(2-Oxopyrrolidin-1-yl)butyramide;Levetiracetam
CAS NO.:102767-28-2
Empirical Formula: C8H14N2O2
Molecular Weight: 170.209
MDL number: MFCD03265610
EINECS: 600-348-9
| Pack Size | Price | Stock | Quantity |
| 250MG | RMB31.20 | In Stock |
|
| 1G | RMB43.20 | In Stock |
|
| 5G | RMB86.40 | In Stock |
|
| 25G | RMB237.60 | In Stock |
|
| 100g | RMB779.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 118-119°C |
| alpha | -89.7 º |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: >5mg/mL |
| form | powder |
| color | white |
| optical activity | [α]/D -90±5°, c = 1 in acetone |
| BCS Class | 3 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)/t6-/s3 |
| InChIKey | HPHUVLMMVZITSG-UHFFFAOYSA-N |
| SMILES | C(N)(=O)[C@@H](N1CCCC1=O)CC |&1:3,r| |
| CAS DataBase Reference | 102767-28-2(CAS DataBase Reference) |
Description and Uses
Levetiracetam was first introduced in the US as an adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy. This second-generation analog of piracetam can be prepared by condensation of (S)-2-aminobutyramide with 4-chlorobutyryl chloride. Although its mechanism of action is not well established, it was shown that [3H]-levetiracetam reversibly binds to a specific site predominantly present in the membranes of the brain. Unlike conventional anticonvulsants such as phenytoin, carbamazepine, valproic acid, phenobarbital, diazepam and clonazepam, compounds structurally-related to levetiracetam, such as piracetam and aniracetam, also have affinity for this site. Levetiracetam reveals a broad and unique profile in animal seizure models, including promising antiepileptogenic properties. Besides being rapidly and almost completely absorbed in man (oral bioavailability>95%), it possesses a favorable pharmacokinetic profile since it is not hepatically metabolized but only partly hydrolized into the inactive carboxylic acid by enzymes in a number of tissues including blood cells, it is minimally bound to plasma proteins (<10%) and does not inhibit or induce hepatic enzymes. Therefore levetiracetam has a low potential for drug interaction, providing a useful alternative as adjunctive therapy to treat seizures refractory to conventional anticonvulsants.
Used as adjunctive therapy in the treatment of partial onset seizures in adults and children 4 years of age and older with epilepsy.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H319 |
| Precautionary statements | P301+P312+P330-P305+P351+P338 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 22-36-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-45-36/37-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | UX9656166 |
| HS Code | 29337900 |
| Hazardous Substances Data | 102767-28-2(Hazardous Substances Data) |
| Toxicity | LD50 in male mice, male rats (mg/kg): 1081, 1038 i.v. (Gobert, 1990) |