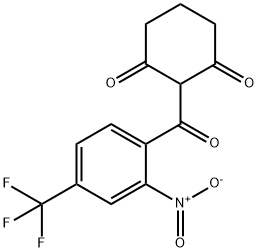
Nitisinone , ≥97% , 104206-65-7
CAS NO.:104206-65-7
Empirical Formula: C14H10F3NO5
Molecular Weight: 329.23
MDL number: MFCD01752192
EINECS: 691-056-0
| Pack Size | Price | Stock | Quantity |
| 250mg | RMB94.40 | In Stock |
|
| 1g | RMB243.20 | In Stock |
|
| 5g | RMB773.60 | In Stock |
|
| 25g | RMB2759.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 129-131°C |
| Boiling point: | 486.2±45.0 °C(Predicted) |
| Density | 1.484±0.06 g/cm3(Predicted) |
| RTECS | GV0766666 |
| storage temp. | -20°C |
| solubility | DMSO: ≥5mg/mL |
| pka | 3.13±0.50(Predicted) |
| form | Solid |
| color | white to brown |
| InChIKey | OUBCNLGXQFSTLU-UHFFFAOYSA-N |
| EPA Substance Registry System | 1,3-Cyclohexanedione, 2-[2-nitro-4-(trifluoromethyl)benzoyl]- (104206-65-7) |
Description and Uses
Nitisinone was originally developed as a pesticide and then launched as an adjunct to dietary restriction of tyrosine and phenylalanine for the treatment of hereditary tyrosinaemia type I. In this inborn error of metabolism, fatal liver disease results either from liver failure during infancy or early childhood or from development of hepatocellular carcinoma during childhood or adolescence. This is caused by accumulation of toxic metabolites due to deficiency of fumarylacetoacetase, the last enzyme of the tyrosine catabolic pathway. Nitisinone, which acts as an inhibitor of the 4-hydroxyphenylpyruvate dioxygenase, prevents the formation of toxic metabolites such as succinylacetoacetate in the liver. Administration of a single dose of nitisinone in mice showed a rapid, significant and persistent inhibition of 4-hydroxyphenylpyruvate dioxygenase. In a murine model of tyrosinaemia type I, administration of nitisinone abolished acute liver failure. Additional dietary tyrosine restriction in the same model on long term follow-up (> 2 years) showed complete correction of liver function tests and succinylacetone levels, and cancer-free survival improvements when compared to historical controls. In healthy volunteers, nitisinone was well tolerated, peak plasma concentrations were rapidly attained following a single dose of 1 mglkg and the half-life time was approximately 54 h. Following the administration of nitisinone (1 mg/kg), the concentrations of tyrosine in plasma increased, were still 8 times those of background at 14 days after dosing, but had returned to background levels within 2 months of the second dose. Elevated tyrosine levels are a potential risk of cornea1 opacities. No treatment related comeal lesions were seen after administration of high dose of nitisinone in mice. In children diagnosed when they were less than 2 months old, when nitisinone treatment was combined with a restricted diet, the four-year survival rate was 88%, compared to 29% from historical data of children treated with restricted diet alone. So, there is some clear evidence that nitisinone treatment associated with restricted diet can reduce the risk of early hepatocellular carcinoma when started before two years of age. On the contrary, in patients with late start of nitisinone treatment there is a considerable risk of liver malignancy. Even if 10% of patients have not clinically responded to nitisinone, studies have shown that oral nitisinone treatment plus dietary restriction has greatly improved the survival of patients and reduced the need of liver transplantation during early childhood.
Nitisinone is a herbicidal triketone that inhibits 4-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme involved in plastoquinone biosynthesis in plants and in tyrosine catabolism in mammals. It is used in treatment of inherited tyrosinemia type I.
Safety
| Symbol(GHS) |   GHS07,GHS06 |
| Signal word | Danger |
| Hazard statements | H311-H302 |
| Precautionary statements | P280-P302+P352-P312-P322-P361-P363-P405-P501-P264-P270-P301+P312-P330-P501 |
| WGK Germany | 3 |