Chloromethane solution , 100mg/L, dissolved in blowing-catch methanol solution , 74-87-3
Synonym(s):
Methyl chloride
CAS NO.:74-87-3
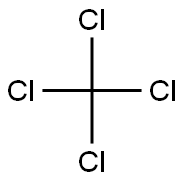
Empirical Formula: CH3Cl
Molecular Weight: 50.49
MDL number: MFCD00000872
EINECS: 200-817-4
PRODUCT Properties
| Melting point: | −97 °C(lit.) |
| Boiling point: | −24.2 °C(lit.) |
| Density | 0.915 g/mL at 25 °C(lit.) |
| vapor density | 1.74 (vs air) |
| vapor pressure | 3796 mm Hg ( 20 °C) |
| refractive index | 1.0007 |
| Flash point: | <-30 °F |
| storage temp. | 2-8°C |
| solubility | water: soluble5.32g/L at 25°C |
| form | Colorless gas |
| color | Colorless to Almost colorless |
| Odor | faint sweet ethereal odor |
| explosive limit | 19% |
| Water Solubility | 5.347g/L(24.9 ºC) |
| Merck | 14,6041 |
| BRN | 1696839 |
| Henry's Law Constant | In seawater: 5.22 at 5 °C, 6.36 at 10 °C, 8.72 at 15 °C, 9.35 at 20 °C, 11.20 at 25 °C (Moore,
2000) |
| Exposure limits | TLV-TWA 50 ppm (~105 mg/m3) (ACGIH),
100 ppm (~210 mg/m3) (OSHA); ceiling
100 ppm (MSHA), 200 ppm (OSHA); TLV STEL 100 ppm (ACGIH); carcinogenicity:
Animal Inadequate Evidence, Human Inad equate Evidence (IARC). |
| Dielectric constant | 12.6(-20℃) |
| Stability: | Stable. May react violently or explosively with interhalogens, magnesium, zinc, potassium, sodium or their alloys. Incompatible with natural rubber and neoprene composites, but does not attack PVA. Highly flammable. May decompose upon exposure to moist air or water. |
| CAS DataBase Reference | 74-87-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 41, Sup 7, 71) 1999 |
| EPA Substance Registry System | Chloromethane (74-87-3) |
Description and Uses
Methyl chloride is a colorless, flammable gas
with a faintly sweet, nonirritating odor at room
temperature. It is shipped as a transparent liquid
under its vapor pressure of about 59 psig at
70°F (407 kPa at 21.1℃).
Methyl chloride burns feebly in air, but forms
mixtures with air that can be explosive within its
flammability range.
Dry methyl chloride is very stable at normal
temperatures and in contact with air. In the
presence of moisture, it hydrolyzes slowly,
which results in the formation of corrosive hydrochloric
acid. At temperatures above 700°F
(371℃), methyl chloride may decompose into
toxic end-products (hydrochloric acid, phosgene,
chlorine, and carbon monoxide). It is
slightly soluble in water and very soluble in
alcohol, mineral oils, chloroform, and most organic
liquids.
Methyl chloride is used as a refrigerant,as a local anesthetic, as a blowing agentfor polystyrene foams, and as a methylat ing agent in the synthesis of a number ofchemicals of commercial application.
Safety
| Symbol(GHS) |     GHS02,GHS04,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H221-H280-H351-H361fd-H373-H420 |
| Precautionary statements | P202-P210-P260-P308+P313-P410+P403-P502 |
| Hazard Codes | F+,Xn,T,F |
| Risk Statements | 12-40-48/20-67-66-22-19-38-23/25-11-39/23/24/25-23/24/25-62-63 |
| Safety Statements | 9-16-33-29-36-24-45-7-36/37 |
| RIDADR | UN 1993 3/PG 1 |
| WGK Germany | 2 |
| RTECS | PA6300000 |
| Autoignition Temperature | 1169 °F |
| HS Code | 2903.11.0010 |
| DOT Classification | 2.1 (Flammable gas) |
| HazardClass | 2.1 |
| PackingGroup | II |
| Hazardous Substances Data | 74-87-3(Hazardous Substances Data) |
| Toxicity | LC50 (inhalation) for mice 3,146 ppm/7-h, rats 152,000 mg/m3/30-min (quoted, RTECS, 1985). |
| IDLA | 2,000 ppm |