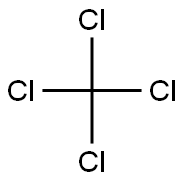
Carbon tetrachloride standard solution , analyticalstandard,1000ug/mlinmethanol , 56-23-5
Synonym(s):
ACT-2;Carbon tetrachloride solution;CCL4;Tetrachloromethane;Tetrachloromethane in dimethyl sulfoxide
CAS NO.:56-23-5
Empirical Formula: CCl4
Molecular Weight: 153.82
MDL number: MFCD00000785
EINECS: 200-262-8
PRODUCT Properties
| Melting point: | -23 °C |
| Boiling point: | 76-77 °C(lit.) |
| Density | 1.594 g/mL at 25 °C(lit.) |
| vapor density | 5.32 (vs air) |
| vapor pressure | 4.05 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −2 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with ethanol, benzene, chloroform, ether, carbon disulfide (U.S. EPA, 1985), petroleum
ether, solvent naphtha, and volatile oils (Yoshida et al., 1983a). |
| form | Liquid |
| color | Clear colorless |
| Relative polarity | 0.052 |
| Odor | Ethereal, sweet, pungent odor detectable at 140 to 584 ppm (mean = 252 ppm) |
| Odor Threshold | 4.6ppm |
| biological source | rat |
| Water Solubility | 0.8 g/L (20 ºC) |
| λmax | λ: 265 nm Amax: 1.0 λ: 270 nm Amax: 0.30 λ: 280 nm Amax: 0.07 λ: 290 nm Amax: 0.02 λ: 300-400 nm Amax: 0.01 |
| Merck | 13,1826 |
| BRN | 1098295 |
| Henry's Law Constant | 2.15 at 30 °C (headspace-GC, Sanz et al., 1997) |
| Exposure limits | NIOSH REL: STEL 1 hour 2 ppm, IDLH 200 ppm; OSHA PEL: TWA
10 ppm, C 25 ppm, 5-minute/4-hour peak 200 ppm; ACGIH TLV: TWA 5 ppm. |
| Dielectric constant | 2.2(20℃) |
| InChIKey | VZGDMQKNWNREIO-UHFFFAOYSA-N |
| LogP | 2.830 |
| Surface tension | 26.95mN/m at 20°C |
| Surface tension | 26.15mN/m at 298.15K |
| CAS DataBase Reference | 56-23-5(CAS DataBase Reference) |
| IARC | 2B (Vol. 20, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Carbon tetrachloride(56-23-5) |
| EPA Substance Registry System | Carbon tetrachloride (56-23-5) |
Description and Uses
Carbon tetrachloride is a manufactured chemical and does not occur naturally in the environment. It is produced by chlorination of a variety of low molecular weight hydrocarbons such as carbon disulfide, methane, ethane, propane, or ethylene dichloride and also by thermal chlorination of methyl chloride. Carbon tetrachloride is a precursor for chlorofluorocarbon (CFC) gases that have been used as aerosol propellant. A decrease in this use is occurring due to the agreement reached in the Montreal Protocol for the reduction of environmental concentrations of ozone-depleting chemicals, including carbon tetrachloride.
In the manufacture of chlorofluorocarbons, which in turn are primarily used as refrigerants; formerly used widely as a solvent, also as a grain fumigant and in fire extinguishers. Because of toxicity consumer uses have been discontinued and only industrial use remains.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301+H311+H331-H317-H351-H372-H412-H420 |
| Precautionary statements | P273-P280-P301+P310-P302+P352+P312-P304+P340+P311-P502 |
| Hazard Codes | T,N,F |
| Risk Statements | 23/24/25-40-48/23-52/53-59-39/23/24/25-11-43 |
| Safety Statements | 23-36/37-45-59-61-16-7 |
| OEL | STEL: 2 ppm (12.6 mg/m3) [60-minute] |
| RIDADR | UN 1846 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | FG4900000 |
| F | 8-9 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 29031400 |
| Hazardous Substances Data | 56-23-5(Hazardous Substances Data) |
| Toxicity | LC50 for mice: 9528 ppm (Svirbely); LD50 in rats, mice, dogs (g/kg): 2.92, 12.1-14.4, 2.3 orally; LD50 in mice (g/kg): 4.1 i.p., 30.4 s.c. (IARC, 1979) |
| IDLA | 200 ppm |