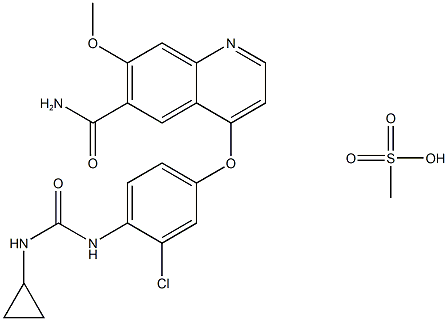
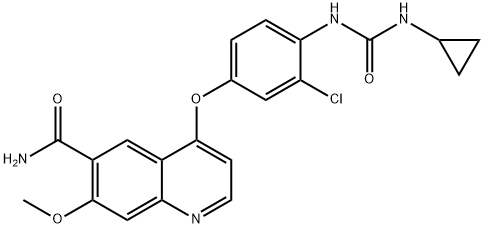
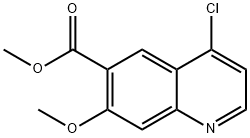
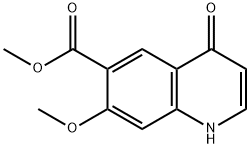
lenvatinibMesylate , 99% , 857890-39-2
Synonym(s):
4-[3-Chloro-4-(N’-cyclopropylureido)phenoxy]-7-methoxyquinoline-6-carboxamide methanesulfonate;4-[3-Chloro-4-[(cyclopropylaminocarbonyl)amino]phenoxy]-7-methoxy-6-quinolinecarboxamide mesylate;4-[3-chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-Quinolinecarboxamide methanesulfonate (1:1);Lenvatinib methanesulfonate
CAS NO.:857890-39-2
Empirical Formula: C22H23ClN4O7S
Molecular Weight: 522.959
MDL number:
EINECS: 812-398-0
| Pack Size | Price | Stock | Quantity |
| 250mg | RMB397.60 | In Stock |
|
| 1g | RMB1215.20 | In Stock |
|
| 5g | RMB4799.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >220°C (dec.) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| InChIKey | HWLFIUUAYLEFCT-UHFFFAOYSA-N |
| SMILES | O=C(N)C1C=C2C(N=CC=C2OC2C=C(Cl)C(NC(=O)NC3CC3)=CC=2)=CC=1OC.CS(=O)(O)=O |
Description and Uses
Lenvatinib mesylate (lenvatinib) is an oral targeted therapy medicine known as a receptor‐type tyrosine kinase inhibitor. It is a small-molecule drug (a drug that can enter cells easily), which was developed at Eisai in 2015. It was approved by the FDA in 2015 for the treatment of differentiated thyroid cancer that is either locally recurrent, metastatic, or progressive and did not respond to radioactive iodine treatment. In May 2016, the FDA approved the drug as a combination therapy with everolimus for the treatment of advanced renal cell carcinoma. Because VEGF (and fibroblast growth factor receptors, known as FGFRs) are thought to play a role in cardiovascular signaling pathways, VEGF2R and FGFR inhibition are thought to be the mechanisms behind the primary side effect of lenvatinib mesylate, which is hypertension.
Lenvatinib mesylate is used in cancer:(1) Endometrial carcinoma that is advanced and got worse after other systemic therapies. It is used with pembrolizumab in patients whose cancer is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) and cannot be treated with surgery or radiation therapy.(2) Hepatocellular carcinoma (a type of liver cancer). It is used as the first treatment in patients whose cancer cannot be removed by surgery.(3) Renal cell carcinoma (a type of kidney cancer) that is advanced(4)Thyroid cancer in certain patients whose cancer got worse, came back, or has spread to other parts of the body and did not respond to treatment with radioactive iodine.(5) Lenvatinib Mesylate is used in preparation of anti-human CTLA4xPD-1 bispecific antibodies for diagnosis, prevention and treatment of tumor or anemia.
Lenvatinib mesylate is also being studied in the treatment of other types of cancer.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |