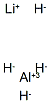Lithium Aluminum Hydride (Powder) , >95.0%(T) , 16853-85-3
Synonym(s):
LAH;LAH, Lithium alanate, Aluminium lithium hydride;Lithium alanate;Lithium aluminium hydride;Lithium tetrahydroaluminate
CAS NO.:16853-85-3
Empirical Formula: LiAlH4
Molecular Weight: 37.9543
MDL number: MFCD00011075
EINECS: 240-877-9
PRODUCT Properties
| Melting point: | 125 °C (dec.)(lit.) |
| Boiling point: | 0°C |
| Density | 0.97 g/mL at 20 °C |
| bulk density | 400kg/m3 |
| Flash point: | 99 °F |
| storage temp. | Store below +30°C. |
| solubility | reacts with H2O, ethanol; seth, tetrahydrofuran |
| form | tablets (~0.5 g each) |
| Specific Gravity | 0.917 |
| color | White to light gray |
| Odor | Odorless solid |
| Water Solubility | Reacts |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,350 |
| Exposure limits | TLV-TWA 2 mg(Al)/m3 (ACGIH). |
| Stability: | Stable. Reacts violently with water, liberating hydrogen. Incompatible with strong oxidizing agents, alcohols, acids. |
| CAS DataBase Reference | 16853-85-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Lithium tetrahydroaluminate(16853-85-3) |
| EPA Substance Registry System | Lithium aluminum hydride (16853-85-3) |
Description and Uses
Lithium aluminum hydride (LiAlH4) is a promising compound for hydrogen storage, with a high gravimetric and volumetric hydrogen density and a low decomposition temperature. Similar to other metastable hydrides, LiAlH4 does not form by direct hydrogenation at reasonable hydrogen pressures; therefore, there is considerable interest in developing new routes to regenerate the material from the dehydrogenated products LiH and Al. It can also be used as a reducing agent in the preparation of reduced graphene oxide (rGO).
Lithium aluminum hydride is among the most important industrial reducingagents. It is used extensively in organic syntheses and also in catalytichydrogenation. Reactant or reagent for:
1. The preparation of thermoplastic polyester polyamides from oleic acid
2. Lithium-polymer batteries
3. Hydrodefluorination of gem-difluoromethylene derivatives
4. Asymmetric aldol reactions
5. Synthesis of Li-Al-N-H composites with hydrogen absorption / desorption properties
6. LAH is a powerful reducing agent for many different reduction reactions such as that of ketones to alcohols
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H260-H314 |
| Precautionary statements | P223-P231+P232-P260-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | F,C,Xi,Xn,F+,T |
| Risk Statements | 15-34-14/15-11-36/37-19-40-10-67-66-22-12-35-37-65-48/20-63-36/38-61-60 |
| Safety Statements | 43-7/8-6A-45-43B-36/37/39-33-26-16-24/25-27-29-62-53 |
| RIDADR | UN 3399 4.3/PG 1 |
| WGK Germany | 2 |
| RTECS | BD0100000 |
| F | 10-21 |
| TSCA | Yes |
| HS Code | 2850 00 20 |
| HazardClass | 4.3 |
| PackingGroup | I |
| Hazardous Substances Data | 16853-85-3(Hazardous Substances Data) |
| Toxicity | TLV-TWA (ACGIH) 2 mg (Al)/m3 |