S6116349
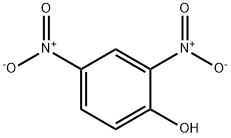
2,4-Dinitrophenol , moistenedwithwater,≥98.0% , 51-28-5
Synonym(s):
α-Dinitrophenol;2,4-DNP;DNP
CAS NO.:51-28-5
Empirical Formula: C6H4N2O5
Molecular Weight: 184.11
MDL number: MFCD00007115
EINECS: 200-087-7
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 108-112 °C (lit.) |
| Boiling point: | 318.03°C (rough estimate) |
| Density | 1,683 g/cm3 |
| vapor density | 6.35 (vs air) |
| vapor pressure | 39(x 10-5 mmHg) at 20 °C (Schwarzenbach et al., 1988) |
| refractive index | 1.4738 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Solubility Sparingly soluble in water; soluble in ethanol, benzene |
| pka | 3.96(at 15℃) |
| form | crystals |
| color | Light yellow |
| PH Range | 2.8(colourless)-4.7(yellow) |
| Odor | Sweet, musty |
| Water Solubility | 0.6 g/100 mL (18 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,3280 |
| BRN | 1246142 |
| Henry's Law Constant | 5.70 x 10-8(atm?m3/mol) at 5 °C (average derived from six field experiments, Lüttke and Levsen, 1997) |
| Stability: | Stable. Combustible. |
| Major Application | Display device, recording materials, inks, paints, method for preserving food, method for gene expression profiling, treatment of parasitic diseases, neurological diseases, epilepsy, cancer, keratin materials, neoplasms, infectious diseases, neutropenia, detecting chromosome aberrations, bacteria in gastrointestinal track |
| CAS DataBase Reference | 51-28-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2,4-dinitro-(51-28-5) |
| EPA Substance Registry System | 2,4-Dinitrophenol (51-28-5) |
Description and Uses
AgriculturalChemical; Drug, Mutagen; Reproductive Effector; HumanData; Primary Irritant. 2,4-DNP is used as an intermediatefor making dyes, photochemicals, pest control agents, woodpreservatives, explosives, chemical indicators, photographicdevelopers, and also in chemical synthesis.
2,4-Dinitrophenol (DNP) can be used:
- As a reactant for catalytic reduction reactions.
- To activate carboxylic acids by converting them into dinitrophenyl (DNP) esters.
- To prepare the corresponding ester via acylation reaction using isobutyric anhydride catalyzed by hafnium triflate.
- As an effective cocatalyst to accelerate the activity and enantioselectivity of primary amine organocatalyst derived from natural primary amino acids for direct asymmetric aldol reaction.
- As an alternative activator to tetrazoles in the reaction of phosphoroamidites with nucleosides.
Safety
| Symbol(GHS) |     GHS02,GHS06,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H208-H300-H311+H331-H372-H400 |
| Precautionary statements | P210-P212-P230-P233-P280-P301+P310-P371+P380+P375-P501 |
| Hazard Codes | T,N,Xi,F |
| Risk Statements | 23/24/25-33-50-39/23/24/25-11-52/53-1 |
| Safety Statements | 28-37-45-61-28A-36/37-16-7-35 |
| RIDADR | UN 1320 4.1/PG 1 |
| WGK Germany | 3 |
| RTECS | SL2800000 |
| TSCA | Yes |
| HazardClass | 4.1 |
| PackingGroup | I |
| HS Code | 29089990 |
| Hazardous Substances Data | 51-28-5(Hazardous Substances Data) |
| Toxicity | LD50 (subcutaneous) for rats 25 mg/kg (quoted, RTECS, 1985). |