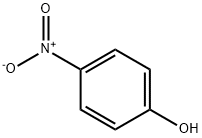
6-Nitrophenol solution , analyticalstandard,1.00mg/mlinmethanol , 100-02-7
Synonym(s):
p-Nitrophenol;p-Nitrophenol solution;4-Nitrophenol;Paracetamol Impurity F;p-Nitrophenol, 1-Hydroxy-4-nitrobenzol
CAS NO.:100-02-7
Empirical Formula: C6H5NO3
Molecular Weight: 139.11
MDL number: MFCD00007331
EINECS: 202-811-7
PRODUCT Properties
| Melting point: | 112 °C |
| Boiling point: | 279 °C(lit.) |
| bulk density | 550kg/m3 |
| Density | 1,27 g/cm3 |
| vapor pressure | 0.6 mm Hg ( 120 °C) |
| refractive index | 1.5723 (estimate) |
| Flash point: | 169 °C |
| storage temp. | 2-8°C |
| solubility | ethanol: soluble95%, clear, dark yellow (100 mg/mL) |
| form | Crystalline Powder, Crystals and/or Chunks |
| pka | 7.15(at 25℃) |
| color | Yellow to brown |
| Specific Gravity | 1.479 |
| Odor | Odorless |
| PH Range | 5.3(colorless)-7.6(Yellow) |
| PH | 4.4 (5g/l, H2O, 24℃)(anhydrous substance) |
| Water Solubility | 1.6 g/100 mL (25 ºC) |
| Sensitive | Light Sensitive |
| λmax | 320nm, 405nm |
| Merck | 14,6620 |
| BRN | 1281877 |
| Henry's Law Constant | 1.63 x 10-7 at 5 °C (average derived from six field experiments, Lüttke and Levsen, 1997) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases, organics, combustible material, reducing agents. Combustible. |
| Major Application | Display device, solar cells, nanoparticles, electrolytic capacitors, lithographic printing plate, leak detection method, falsification-proof security paper, correction fluid, detergent, fertilizer, identifying fresh and stale rice, diapers, detecting lactic acid bacteria, drug delivery, evaluating dental caries activity |
| CAS DataBase Reference | 100-02-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-nitro-(100-02-7) |
| EPA Substance Registry System | p-Nitrophenol (100-02-7) |
Description and Uses
4-Nitrophenol (also called p-nitrophenol or 4-hydroxynitrobenzene) is a phenolic compound that has a nitro group at the opposite position of hydroxyl group on the benzene ring. 4-Nitrophenol shows two polymorphs in the crystalline state. The alpha form is colorless pillars, unstable at room temperature, and stable toward sunlight. The beta form is yellow pillars, stable at room temperature, and gradually turns red upon irradiation of sunlight. Usually 4-nitrophenol exists as a mixture of these two forms. Generally, 4-nitrophenol is used in manufacturing of drugs (e.g., acetaminophen), fungicides, methyl and ethyl parathion insecticides, and dyes, and to darken leather.
4-Nitrophenol is used in dyestuff and pesticide synthesis, as a
fungicide, bactericide, and wood preservative, as a chemical
indicator, and as a substrate for experiments on cytochrome
P450 2E1.
4-Nitrophenol is used as a precursor to prepare phenetidine,
acetophenetidne and pH indicator. Its carboxylate ester derivatives are
involved as an active component for the construction of amide moieties
in peptide synthesis. It is used as an intermediate in the synthesis of
paracetamol and N-acetyl-p-aminophenol dyestuffs.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302+H312+H332-H373 |
| Precautionary statements | P260-P280-P301+P312-P302+P352+P312-P304+P340+P312-P314 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 20/21/22-33-39/23/24/25-23/24/25-11 |
| Safety Statements | 28-28A-45-36/37-16-7 |
| RIDADR | UN 1663 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | SM2275000 |
| F | 8 |
| Autoignition Temperature | 541 °F |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29089000 |
| Hazardous Substances Data | 100-02-7(Hazardous Substances Data) |
| Toxicity | LD50 orally in mice, rats: 467, 616 mg/kg, K.C. Back et al., Reclassification of Materials Listed as Transportation Health Hazards (TSA-20-72-3; PB214-270, 1972) |