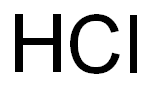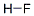puriss.,24.5-26.0% , 7647-01-0
Synonym(s):
Hydrochloric acid;Hydrochloric acid solution;Hydrogen chloride solution;Muriatic Acid, 23 deg. Be
CAS NO.:7647-01-0
Empirical Formula: ClH
Molecular Weight: 36.46
MDL number: MFCD03457719
EINECS: 231-595-7
PRODUCT Properties
| Melting point: | -35 °C |
| Boiling point: | >100 °C (lit.) |
| Density | 1.2 g/mL at 25 °C (lit.) |
| vapor density | 1.3 (vs air) |
| vapor pressure | 613 psi ( 21.1 °C) |
| Flash point: | 10℃ (tag closed test) |
| refractive index | 1.3535 |
| storage temp. | Store at +2°C to +25°C. |
| solubility | H2O: soluble |
| form | liquid |
| pka | -7(at 25℃) |
| color | Light Yellow |
| Specific Gravity | 1.19 |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution); |
| Odor | Sharp, irritating odor detectable at 0.25 to 10 ppm |
| explosive limit | 2-13.4% (v/v) 2-Propanol) |
| biological source | synthetic |
| Water Solubility | miscible |
| Sensitive | Air & Light Sensitive |
| Merck | 14,4780 |
| Exposure limits | Ceiling limit 5 ppm (~
7 mg/m3). |
| Dielectric constant | 4.6(20℃) |
| Stability: | Stable. Incompatible with alkalies, most metals. Avoid contact with water. |
| CAS DataBase Reference | 7647-01-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 54) 1992 |
| NIST Chemistry Reference | Hydrogen chloride(7647-01-0) |
| EPA Substance Registry System | Hydrochloric acid (7647-01-0) |
Description and Uses
A water solution of hydrogen chloride of varied concentrations. It is a clear, colorless or slightly yellowish, corrosive liquid having a pungent odor. It is miscible with water and with alcohol. Concentrations of hydrochloric acid are expressed in percent by weight, or may be expressed in Baume degrees (Be0) from which percentages of hydrochloric acid and specific gravities may readily be derived. The usually available concentrations are 18°, 20°, 22°, and 23° Be. Concentrations above 13° Be (19.6%) fume in moist air, lose hydrogen chloride, and create a corrosive atmosphere. Because of these characteristics, suitable precautions must be observed during sampling and analysis to prevent losses. Note: Hydrochloric acid is produced by various methods that might impart trace amounts of organic compounds as impurities. The manufacturer, vendor, or user is responsible for identifying the specific organic compounds that are present and for meeting the requirements for organic compounds. Methods are provided for their determination. In applying the procedures any necessary standards should be used to quantitate the organic compounds present in each specific product.
Hydrochloric acid is one of the most widely used acids and a common laboratory reagent. It is used in the manufacture of chlorides, in the pickling and cleaning of metal products, as a processing agent for manufacturing various food products, as a cleaning agent, in organic synthesis, and for neutralizing alkalies.
Hydrogen chloride is a fire-effluent gas.Firefighters are frequently exposed to significant concentrations of HCl (Brandt-Raufet al. 1988). Large amounts of HCl arereleased from the oxidative thermal degradation of polyvinyl chloride (PVC)-derivedfiberglass, cotton, and jute brattices in mines.At 250°C (482°F) its concentration is foundto be >5 ppm (De Rosa and Litton 1986).The gas is absorbed by water droplets,entrapped in soot particles, causing risk ofexposure of the acid to the eyes, throat,and lungs of mine workers. Stack emissionsof HCl can result from burning plastic-richwastes (e.g., hospital wastes) (Powell 1987).Emissions of 1.0–1.6 g HCl/kg waste havebeen reported (Allen et al. 1986)..
Safety
| Symbol(GHS) |  GHS05 |
| Signal word | Warning |
| Hazard statements | H290 |
| Precautionary statements | P234-P390 |
| Hazard Codes | T,C,F,Xi,F+,Xn |
| Risk Statements | 36/37/38-37-34-35-23-20-11-67-66-22-19-12-10-40-20/22-39/23/24/25-23/24/25-41-37/38 |
| Safety Statements | 26-45-36/37/39-9-33-29-16-46-36/37-39 |
| RIDADR | UN 2924 3/PG 2 |
| OEL | Ceiling: 5 ppm (7 mg/m3) |
| WGK Germany | 2 |
| RTECS | MW4025000 |
| F | 3 |
| Autoignition Temperature | 425°C (2-Propanol) |
| TSCA | Yes |
| HS Code | 2806 10 00 |
| DOT Classification | 2.3, Hazard Zone C (Gas poisonous by inhalation) |
| HazardClass | 3 |
| PackingGroup | I |
| Hazardous Substances Data | 7647-01-0(Hazardous Substances Data) |
| Toxicity | LC50 (30 min) in mice, rats: 2142, 5666 ppm (Darmer) |
| IDLA | 50 ppm |