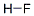Hydrofluoric acid , Electronic grade, 49WT.%InH2O, ≥99.99998%MetalsBasis , 7664-39-3
Synonym(s):
HF;Hydrofluoric acid solution, Fluoric acid;Hydrogen Fluoride
CAS NO.:7664-39-3
Empirical Formula: FH
Molecular Weight: 20.01
MDL number: MFCD00011346
EINECS: 231-634-8
PRODUCT Properties
| Melting point: | -35°C |
| Boiling point: | 105°C |
| Density | 1.15 g/mL at 25 °C(lit.) |
| vapor density | 1.27 (vs air) |
| vapor pressure | 25 mm Hg ( 20 °C) |
| Flash point: | 112°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | very soluble in H2O, ethanol; soluble in ethyl ether |
| pka | 3.17(at 25℃) |
| form | Liquid, Double Sub-Boiling Quartz Distillation |
| Specific Gravity | 1.15 |
| color | APHA: ≤10 |
| PH Range | 1 |
| PH | 3.27(1 mM solution);2.65(10 mM solution);2.12(100 mM solution) |
| Odor | Acrid, irritating odor |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4790 |
| Exposure limits | Ceiling limit 3 ppm (~2.5 mg/m3) as F
(ACGIH); TWA 3 ppm (MSHA and OSHA). |
| Dielectric constant | 17.0(-73℃) |
| Stability: | Stable. Hygroscopic. Incompatible with glass, alkali metals, light metals, alkaline earth metals |
| LogP | 0.1 at 20℃ |
| CAS DataBase Reference | 7664-39-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen fluoride(7664-39-3) |
| EPA Substance Registry System | Hydrofluoric acid (7664-39-3) |
Description and Uses
Hydrofluoric acid is a solution of hydrogen fluoride in water. Hydrofluoric acid is highly corrosive inorganic acid. It is utilized widely in the manufacture of ceramics and graphite, in the electropolishing and pickling of metals, in the etching and frosting of glass, in the semiconductor industry as etchant and cleaning agent, in the chemical and oil-refining industries, and in cleaning solutions, laundry powder and pesticides. Hydrofluoric acid is also widely used in the preparation of many useful fluorine compounds, such as Teflon, Freon, fluorocarbons, and many medications such as fluoxetine (Prozac).
Diluted hydrofluoric acid (1-3 %wt) is used in the "Oil Patch" in a mixture with other acids (HCl or organic acids) in order to stimulate the production of water, oil and gas wells specifically where sandstone is involved. HF is also used in oil refining. In a standard oil-refinery process known as Alkylation, isobutane is alkylated with low-molecular weight alkenes (primarily a mixture of propylene and butylene) in the presence of a strong acid catalyst, hydrofluoric acid. The catalyst is able to protonate the alkenes (propylene, butylene) to produce reactive carbo-cations , which cause alkylation of isobutane. The phases separate spontaneously, so the acid phase is vigorously mixed with the hydrocarbon phase to create sufficient contact surface. Hydrofluoric acid (HF) is used principally in organofluorine chemistry. Additionally, most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6, (Cryolite), and AlF3(aluminum trifluoride). A molten mixture of these solids serves as a high-temperature solvent for the production of metallic aluminum. Concerns about fluorides in the environment have led to a search for alternative technologies. Other inorganic fluorides prepared from hydrofluoric acid include NaF and UF6.
The ability of hydrofluoric acid to dissolve metal
oxides is the basis of several applications. It removes
oxide impurities from stainless steel in a process called
pickling. Surface oxides are removed from silicon with
hydrofluoric acid in the Semiconductor Industry.
For this purpose, dilute hydrofluoric acid is sold as
a household rust stain remover. Recently, it has even
been used in “Car-Wash” facilities in “wheel cleaner”
compounds.
Due to its ability to dissolve oxides, hydrofluoric acid
is useful for dissolving rock samples (usually powdered)
prior to analysis. Similarly, this acid has been used to
extract organic fossils from silicate rocks. Fossiliferous
rock may be immersed directly into the acid, or a cellulose
nitrate film may be applied (dissolved in amyl
acetate), which adheres to the organic component and
allows the rock to be dissolved around it.
Safety
| Symbol(GHS) |   GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H300+H310+H330-H314 |
| Precautionary statements | P260-P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T+,C,T,Xn |
| Risk Statements | 26/27/28-35-36/37/38-20/21/22 |
| Safety Statements | 26-36/37/39-45-7/9-36/37-28-36 |
| OEB | B |
| OEL | TWA: 3 ppm (2.5 mg/m3), Ceiling: 6 ppm (5 mg/m3) [15-minute] |
| RIDADR | UN 1790 8/PG 2 |
| WGK Germany | 2 |
| RTECS | MW7875000 |
| Hazard Note | Corrosive |
| TSCA | Yes |
| DOT Classification | 8, Hazard Zone C (Corrosive material) |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28111100 |
| Hazardous Substances Data | 7664-39-3(Hazardous Substances Data) |
| Toxicity | LC50 (15 min.) in rats, guinea pigs: 2689, 4327 ppm (Rosenholtz) |
| IDLA | 30 ppm |