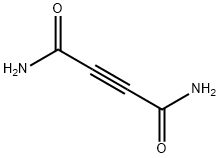
PRODUCT Properties
| Melting point: | 179 °C |
| Boiling point: | 209.98°C (rough estimate) |
| Density | 1.4421 (rough estimate) |
| refractive index | 1.4610 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMF: 10 mg/ml; DMSO: 20 mg/ml; DMSO:PBS (pH 7.2) (1:7): 0.12 mg/ml |
| form | Fine Crystalline Powder |
| pka | 13.01±0.50(Predicted) |
| color | Cream to beige |
| EPA Substance Registry System | 2-Butynediamide (543-21-5) |
Description and Uses
Cellocidin is an antibiotic originally isolated from S. chibaensis. It is active against various bacterial strains including M. tuberculosis and against the trypanosomes T. brucei and T. rhodesiense (IC50s = 150 and 30 ng/ml, respectively). It inhibits proliferation of LCL1 and LCL2 cells transformed by Epstein-Barr virus (EBV), activates the c-Myc and NF-κB pathways in BC3 and LCL1 cells, and induces necrotic cell death in B cells infected with gammaherpes virus. Cellocidin (100-200 ppm) is protective against bacterial leaf blight in rice plants and inhibits α-ketoglutarate oxidation in X. oryzae, the bacterium that causes leaf blight, when used at a concentration of 1 ppm, suggesting that it inhibits the citric acid cycle. Formulations containing cellocidin have been used as agricultural pesticides.
Cellocidin is a small neutral alkyne produced by a number of Streptomyces species, first discovered by Suzuki and colleagues in 1958. Cellocidin has a broad antibacterial, antifungal and antitumor profile due to its ability to react with endogenous thiols like cysteine and glutathione. Cellocidin occurs as a weak active in many bioassays using actinomycete crude extracts and is thus a useful standard for chemical and bioassay dereplication.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| Hazard Codes | T |
| Risk Statements | 25-21 |
| Safety Statements | 45-36/37-28A |
| HS Code | 29241900 |
| Toxicity | LD50 i.v. in mice: 11 mg/kg (Suzuki) |