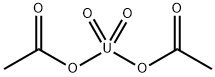
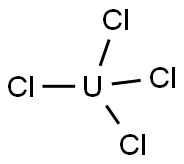
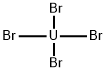
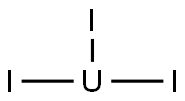PRODUCT Properties
| Melting point: | 314-316°C (dec.) |
| Boiling point: | 4160.06°C (estimate) |
| Density | 1.01 g/mL at 25 °C |
| storage temp. | Refrigerator |
| solubility | Aqueous Base (Slightly) |
| form | silvery-white orthorhombic crystals |
| color | Pale Brown |
| EPA Substance Registry System | Uranium (7440-61-1) |
Description and Uses
Uranium is a silver-white, lustrous, heavy, mildly radioactive metal. Its appearance will
change upon exposure to air or water, as oxidation occurs. Its colour darkens through
brass, from brown to charcoal grey. Powders, fines, chips, or turnings oxidise rapidly,
yielding a dull or flat dark grey or brown colour. Uranium is almost as hard as steel and
much denser than lead. Natural uranium is used to make fuel for nuclear power plants;
depleted uranium is the leftover product. Some alloys will oxidise more slowly, retaining
the silver-white and then brassy colour. No odour is found. Uranium is used as an abundant
source of concentrated energy. Uranium occurs in most rocks in concentrations of 2–4
parts per million and is as common in the Earth’s crust as tin, tungsten, and molybdenum.
Uranium occurs in seawater and can be recovered from the oceans.
Uranium is a naturally occurring radioactive element. Natural uranium is a mixture of three isotopes: 234U, 235U, and 238U. The most common isotope is 238U; it makes up about 99% of natural uranium by mass. Depleted uranium is a mixture of the same three uranium isotopes except that it has very little 234U and 235U. It is less radioactive than natural uranium. The high density of uranium means that it also finds uses in the keels of yachts and as counterweights for aircraft control surfaces, as well as for radiation shielding. Uranium metal is known to react dangerously with carbon tetrachloride, chlorine, fluorine, nitric acid, nitric oxide, selenium, sulphur, and water (in finely divided form). On decomposition with fire, it produces uranium metal fume and/or oxide. Radioactive progenies (daughters), thorium-234, protactinium-234, and protactinium-234m (metastable), are produced by natural radioactive decay; they are the source of the majority of the penetrating radiation. These isotopes can be concentrated in situations where the metal is melted, condensed, or dissolved, potentially elevating the observed external dose rate. Many industries involved in mining, milling, and processing of uranium can also release it into the environment. Inactive uranium industries may continue to release uranium into the environment.
235U in nuclear power reactors and nuclear weapons. Uranium depleted of 235U to manufacture of armor-piercing ammunition, in inertial guidance devices and gyro compasses, as a counterweight for missile reentry vehicles, as radiation shielding material, and x-ray targets.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H300-H330-H373-H413 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P260-P314-P501 |
| Hazard Codes | T+ |
| Risk Statements | 20-34-53-33-26/28 |
| Safety Statements | 26-36/37/39-45-61-20/21 |
| RIDADR | UN 3264 8/PG 3 |
| OEB | C |
| OEL | TWA: 0.2 mg/m3, STEL: 0.6 mg/m3 |
| WGK Germany | 3 |
| HazardClass | 7 |
| PackingGroup | Commercial |
| HS Code | 28441000 |
| Hazardous Substances Data | 7440-61-1(Hazardous Substances Data) |
| Toxicity | Three isotopes (234U, 235U, 238U) exist, and a large number of uranium salts are known. They present both toxic and radiological hazards. The most important use of uranium is in the nuclear energy industry, but uranium compounds are also used in ceramics, as catalysts and in certain alloys. Entry into the body can occur during a variety of processes involved with the mining, processing or use of uranium and its compounds, and is probably largely by inhalation of dusts, fumes, etc. or by ingestion. Acute uranium toxicity is primarily nephrotoxicity. About 50% of plasma uranium is bound, as the uranyl ion, to bicarbonate (HCO23 ), which is filtered by the glomerulus. As a result of acidification in the proximal tubule, the bicarbonate complex dissociates followed by reabsorption of the HCO23 ; the released UO21 then becomes attached to the membrane of the proximal tubule cells. Loss of cell function follows, as evidenced by increased concentration of glucose, amino acids, and proteins in the urine. 2,3-Mercapto-1-propanol (British Anti-Lewisite, BAL) is ineffective as a therapeutic agent for uranium poisoning; CaEDTA is recommended. Chronic uranium toxicity appears to be radiation related, the effects being similar to those of ionizing radiation. In humans, cancer of the lung, bone, and lymphatic system are all known to occur. |
| IDLA | 10 mg U/m3 |