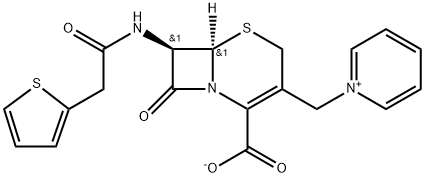
Cephaloridine , 98%+ , 50-59-9
PRODUCT Properties
| Melting point: | 184°C |
| alpha | D +47.7° (c = 1.25 in water) |
| Density | 1.3230 (rough estimate) |
| refractive index | 1.6390 (estimate) |
| storage temp. | 2-8°C |
| pka | 3.2(at 25℃) |
| optical activity | +47.720 (c 1.25, H2O) |
| Water Solubility | >20g/L(21 ºC) |
Description and Uses
This was derived by adding two side chains to the nucleus of cephalothin, and has the formula 7-(2-thienyl acetamido)-3-(1-pyridylmethyl)-3-cephem-4-carboxylic acid betaine (Muggleton et al., 1964). Cephaloridine was initially widely used, but it had two main disadvantages. It was an unreliable antistaphylococcal drug, as it was relatively easily hydrolyzed by Staphylococcus aureus beta-lactamase (Laverdiere et al., 1978; Sabath, 1989). Second, cephaloridine was nephrotoxic (Foord, 1975; Appel and Neu, 1977). Marketing of the drug was discontinued in the USA in December 1980. Nowadays, this drug is used very rarely, if at all.
Antibacterial agent.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-45 |
| Hazardous Substances Data | 50-59-9(Hazardous Substances Data) |
| Toxicity | LD50 mice, rats (g/kg): >15, 2.5-4 orally; in monkeys (g/kg): >0.2 i.m. (Atkinson) |