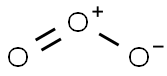
Ozone , 99% , 10028-15-6
PRODUCT Properties
| Melting point: | 193℃ |
| Boiling point: | -110℃ |
| Density | 1.46 g/cm3 |
| vapor pressure | 55kPa at -12℃ |
| solubility | slightly soluble in H2O |
| form | blue gas |
| color | Blue or violet-black solid or unstable colorless gas or dark-blue liquid |
| Odor | Pungent odor, detectable at 0.01 to 0.04 ppm; sharp disagreeable odor at 1 ppm |
| Odor Threshold | 0.0032ppm |
| Water Solubility | 570mg/L at 20℃ |
| Exposure limits | TLV-TWA 0.1 ppm (~0.2 mg/m3) (ACGIH, NIOSH, and MSHA), 0.2 ppm (~0.4 mg/m3) (OSHA); IDHL 10 ppm (NIOSH). |
| Stability: | Unstable - may decompose spontaneously and violently to oxygen. Mixtures containing a moderate partial pressure of ozone, and pure ozone at even low pressures are both potentially explosive. May react very violently with combustible materials and reducing agents, such as organics. Even small quantities of organic material, such as traces of g |
| Cosmetics Ingredients Functions | ORAL CARE |
| LogP | -0.87 at 20℃ |
| EPA Substance Registry System | Ozone (10028-15-6) |
Description and Uses
Ozone in the upper layers of the atmosphere (stratosphere) is formed by the reaction of O2 with the elemental oxygen formed from the splitting of O2 by UV radiation. The ozone layer in the stratosphere, though containing a relatively low amount of O3 relative to O2, absorbs UV radiation and serves to protect Earth fromthe destructive andmutagenic properties of solarUV.Ozone is more unstable than O2 and thus more reactive. Ground-level ozone (troposphere) is formed largely fromthe reactionof the byproducts of the incomplete combustion of fossil fuels with elemental oxygen present. Common industrial pollutants and car exhaust by-products such asnitrogen oxides, sulfur oxides, carbon oxides, and hydrocarbons are photochemically cleaved and then react with the O2 present. Natural sources of tropospheric ozone come from ozone migration from the stratosphere with average concentration of about 10–20 ppb in nonurban areas. Tropospheric ozone can be very harmful to human health, and people with conditions such as emphysema, asthma, bronchitis, and heart conditions are especially susceptible. Health effects from ozone are due to its high reactivity resulting in reactions with biological macromolecules and subsequent cellular damage. In addition, ozone conversion to diatomic oxygen results in the production of free oxygen radicals, which can also cause damage. Due to the harmful health effects of ozone, theUS Environmental Protection Agency (EPA) among other government entities has established exposure limits. The National Ambient Air Quality Standards limit for ozone is 0.075 ppm, taken as the annual fourth-highest dailymaximum8 h concentration, averaged over 3 years. Ozone exceeds its limit more often than all other regulated air chemicals, and is in excess most frequently in California.
Ozone is much more reactive than O2, which makes it a very powerful oxidizing agent.Only fluorine is more reactive. It has many commercial uses. It is a strong oxidizer, particularlyof organic compounds, it is a strong bleaching agent for textiles, oils, and waxes, and it is apowerful germicide. It is also used in the manufacture of paper, steroid hormones, waxes, andcyanide and in the processing of acids.
Ozone produced by electrical discharge is used to purify drinking water and to treatindustrial wastes and sewage. It is also use to deodorize air and kill bacteria by passing dry airthrough special ozone-producing electronic devices.
Safety
| Symbol(GHS) |      GHS03,GHS08,GHS05,GHS09,GHS06 |
| Signal word | Danger |
| Hazard statements | H400-H314-H372-H270-H330-H410 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P273-P391-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P260-P264-P270-P314-P501-P220-P244-P370+P376-P403-P273-P391-P501 |
| OEL | Ceiling: 0.1 ppm (0.2 mg/m3) |
| RIDADR | 1956 |
| TSCA | TSCA listed |
| HazardClass | 2.2 |
| Hazardous Substances Data | 10028-15-6(Hazardous Substances Data) |
| Toxicity | LC50 inhal (rat) 4.8 ppm (4 h) PEL (OSHA) 0.1 ppm (0.2 mg/m3) TLV-TWA (ACGIH) 0.1 ppm (0.2 mg/m3) STEL (ACGIH) 0.3 ppm (0.6 mg/m3) |
| IDLA | 5 ppm |