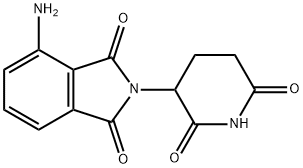
Pomalidomide , 98% , 19171-19-8
Synonym(s):
1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline;3-amino-N-(2,6-dioxo-3-piperidyl)phthalamide;4-Amino-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione;Actimid;IMiD 3
CAS NO.:19171-19-8
Empirical Formula: C13H11N3O4
Molecular Weight: 273.24
MDL number: MFCD12756407
EINECS: 805-902-5
| Pack Size | Price | Stock | Quantity |
| 250mg | RMB38.40 | In Stock |
|
| 1g | RMB83.20 | In Stock |
|
| 5g | RMB236.80 | In Stock |
|
| 10g | RMB436.00 | In Stock |
|
| 25g | RMB860.80 | In Stock |
|
| 100g | RMB2912.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 318.5 - 320.5° |
| Boiling point: | 582.9±45.0 °C(Predicted) |
| Density | 1.570±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥14mg/mL |
| pka | 10.75±0.40(Predicted) |
| form | powder |
| color | yellow |
| Merck | 14,135 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) |
| InChIKey | UVSMNLNDYGZFPF-UHFFFAOYSA-N |
| SMILES | C1(=O)C2=C(C(N)=CC=C2)C(=O)N1C1CCC(=O)NC1=O |
Description and Uses
In February 2013, the US FDA approved pomalidomide (also known as CC4047) for the treatment of multiple myeloma (MM) in patients with disease progression after receiving other cancer therapeutics. Pomalidomide is a 4-amino analog of thalidomide with enhanced potency and an improved toxicity profile. Pomalidomide and thalidomide exert their effects by modulation of immunity, inhibition of angiogenesis, interference with the bone/tumor microenvironment, and inhibition of the cereblon protein. Pomalidomide potently inhibited in vitro proliferation in a variety of human MM cell lines, IC50~10 nM, while thalidomide showed almost no inhibition up to 100 μM. In mouse MM tumor models, 50 mg/kg daily doses of pomalidomide resulted in marked inhibition of tumor growth after 15 days of treatment and complete regression in 3–6 weeks versus thalidomide-treated controls at the same dose. Pomalidomide is prepared by condensation of 4-nitrophthalic anhydride with 3-aminopiperidine-2,6-dione followed by catalytic hydrogenation of the nitro group.
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Danger |
| Hazard statements | H360D |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| WGK Germany | 3 |
| RTECS | NR3397905 |
| HS Code | 29251900 |
| Hazardous Substances Data | 19171-19-8(Hazardous Substances Data) |