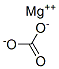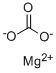PRODUCT Properties
| Melting point: | 990°C |
| Density | 3.050 |
| refractive index | 1.717 |
| solubility | Practically insoluble in water. It dissolves in dilute acids with effervescence. |
| form | Solid |
| color | White |
| Odor | at 100.00?%. odorless |
| Water Solubility | g MgCO3/100g solution at CO2 pressure, kPa, 18°C: 3.5 (203), 4.28 (405), 5.90 (1010), 7.49 (1820), 7.49 (5670); at 0°C 8.58 (3445), at 60°C 5.56 (3445); soluble acids; insoluble alcohol [HAW93] [KIR81] |
| Stability: | Hygroscopic |
| InChI | InChI=1S/CH2O3.Mg/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2 |
| InChIKey | ZLNQQNXFFQJAID-UHFFFAOYSA-L |
| SMILES | [Mg+2].C([O-])(=O)[O-] |
| LogP | -0.809 (est) |
| CAS DataBase Reference | 546-93-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium carbonate(546-93-0) |
| EPA Substance Registry System | Magnesium carbonate (546-93-0) |
Description and Uses
Magnesium carbonate is obtained mainly by mining the natural mineral magnesite. The trihydrate salt, MgCO3·3H2O, is prepared by mixing solutions of magnesium and carbonate ions in the presence of carbon dioxide.
Used in Pharmaceutical units as a Magnesium Salt, Heat Insulator and Refractor, Antacid. Also used in Cosmetics, inks, glass, drying agent, color retention agent and etc.
Safety
| Safety Statements | 24/25-22 |
| OEB | B |
| OEL | TWA: 10 mg/m3 (total) |
| RTECS | OM2470000 |
| HS Code | 28369910 |
| Hazardous Substances Data | 546-93-0(Hazardous Substances Data) |