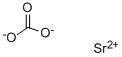Strontium Carbonate , SP , 1633-05-2
Synonym(s):
Strontium carbonate (SrCO);Strontium monocarbonate 3;Strontium(II) carbonate
CAS NO.:1633-05-2
Empirical Formula: CO3Sr
Molecular Weight: 147.63
MDL number: MFCD00011250
EINECS: 216-643-7
| Pack Size | Price | Stock | Quantity |
| 25G | RMB32.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1494 °C (lit.) |
| Density | 3.7 g/mL at 25 °C (lit.) |
| refractive index | 1.518 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | dilute aqueous acid: slightly soluble(lit.) |
| form | Powder |
| Specific Gravity | 3.7 |
| color | white |
| Odor | Odorless |
| PH | 7-8 (20°C, 0.01g/L in H2O) |
| Water Solubility | Soluble in ammonium chloride. Slightly soluble in ammonia and water. |
| Merck | 14,8838 |
| Solubility Product Constant (Ksp) | pKsp: 9.25 |
| Stability: | Stable. Incompatible with strong acids. |
| InChIKey | LEDMRZGFZIAGGB-UHFFFAOYSA-L |
| LogP | -0.809 (est) |
| CAS DataBase Reference | 1633-05-2(CAS DataBase Reference) |
| EPA Substance Registry System | Carbonic acid, strontium salt (1:1) (1633-05-2) |
Description and Uses
Strontium carbonate has the formula of SrCO3 and the molecular weight of 147.6326 g/mol. Strontium carbonate occurs in nature as the mineral “strontianite”. The name strontianite comes from a famous location for the mineral, Strontian, Scotland. Strontianite is strontium carbonate as found naturally. It occurs as white or slightly gray orthorhombic crystals with a refractive index of 1.518. The unit-cell parameters are: a = 5.107 ? , b = 8.414 ? , c = 6.029 ? , Z = 4; V = 259.07 ? 3, Den(Calc) = 3.78. The crystal system is orthorhombic with space group Pmcn and point group 2/m, 2/m, 2/m. Strontium carbonate has only one stable form (aragonite-type structure) and temperature of precipitation has no effect on crystal form, unlike that of calcium or magnesium carbonates.
Used for electronic applications. It is used for manufacturing CTV to absorb electrons resulting from the cathode
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P280-P302+P352-P304+P340 |
| WGK Germany | - |
| TSCA | Yes |
| HS Code | 2836920000 |
| Hazardous Substances Data | 1633-05-2(Hazardous Substances Data) |