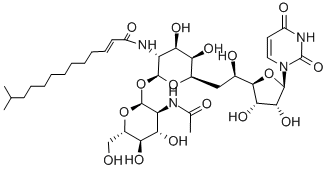
Tunicamycin , 99% , 11089-65-9
Synonym(s):
InSolution Tunicamycin, Streptomyces lysosuperficus - CAS 11089-65-9 - Calbiochem;NSC 177382;Tunicamycin, Streptomyces lysosuperficus - CAS 11089-65-9 - Calbiochem
CAS NO.:11089-65-9
Empirical Formula: C37H60N4O16
Molecular Weight: 816.89
MDL number: MFCD00065709
EINECS: 601-012-4
| Pack Size | Price | Stock | Quantity |
| 50mg | RMB4992.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 234-235℃ (decomposition) |
| alpha | D20 +52° (c = 0.5 in pyridine) |
| Flash point: | 87℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in methanol, ethanol, DMSO, or DMF |
| form | White to off-white solid |
| color | White |
| biological source | Streptomyces sp. |
| Sensitive | Moisture & Light Sensitive |
| BRN | 6888090 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| InChIKey | YJQCOFNZVFGCAF-WPTOCQRYSA-N |
| EPA Substance Registry System | Tunicamycin (11089-65-9) |
Description and Uses
Tunicamycin mixture is a mixture of tunicamycins with variable trans-2,3-unsaturated branched chain fatty acid (BFCA) chain lengths. Tunicamycins are anti-microbial agents that are active against Gram-positive bacteria, fungi, and viruses. They inhibit the N-acetylhexosamine (HexNAc) phosphotransferase family of enzymes in bacteria and prevent peptidoglycan biosynthesis. In eukaryotes, they inhibit N-acetylglucosamine (GlcNAc) phosphotransferase (GPT), preventing the first step in N-linked glycosylation and inducing the unfolded protein response and cell death. The cellular toxicity of tunicamycins is linked to the trans-2,3-unsaturated BCFA, and saturated BCFA-containing tunicamycin derivatives, such as TunR1 and TunR2 , have reduced toxicity. Tunicamycins impair glycosylation of the receptor tyrosine kinases EGFR, HER2, HER3, and IGF-1R, which prevents their translocation out of the endoplasmic reticulum and Golgi apparatus and reduces their protein levels and activity. Tunicamycin sensitizes EGFR inhibitor-resistant U251 glioma and Bx/PC-3 pancreatic adenocarcinoma cells to irradiation.
Tunicamycin has been widely used in the study of glycoprotein synthesis in various biological systems. During protein glycosylation, tunicamycin is noted to be an inhibitor of the transfer of saccharide moieties to dolichol during dolichol-linked glycoprotein synthesis. Dose-dependent inhibition of DNA synthesis may be related to the alteration of glycoproteins, which thereby affects the transport of thymidine into cells. Additionally, tunicamycin has been reported to prevent cell cycle progression in primary cultures of rat glial cells, as well as inhibit lipid-mediated protein glycosylation in chick or mouse fibroblasts in a dose-dependent manner.
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H300 |
| Precautionary statements | P264-P270-P301+P310-P405-P501 |
| Hazard Codes | T+,T |
| Risk Statements | 28 |
| Safety Statements | 28-37/39-45 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | YO7980200 |
| F | 8-10-18-21 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 29419090 |