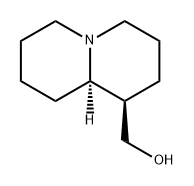
((1R,9aR)-Octahydro-1H-quinolizin-1-yl)methanol , 95% , 486-70-4
CAS NO.:486-70-4
Empirical Formula: C10H19NO
Molecular Weight: 169.26
MDL number: MFCD00213431
EINECS: 207-638-0
| Pack Size | Price | Stock | Quantity |
| 100mg | RMB219.20 | In Stock |
|
| 250mg | RMB372.00 | In Stock |
|
| 1g | RMB1002.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 62-65°C |
| Boiling point: | 160-164°C 4mm |
| alpha | D26 -25.9° (c = 3 in water); D28 -21° (c = 9.5 in alcohol) |
| Density | 0.9660 (rough estimate) |
| refractive index | 1.4610 (estimate) |
| Flash point: | 160-164°C/4mm |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethanol (Slightly, Sonicated), Methanol (Slightly) |
| form | Very Dark Orange |
| pka | 14.90±0.10(Predicted) |
| color | Light yellow to yellow |
| Merck | 14,5609 |
| BRN | 80447 |
| Stability: | Hygroscopic |
| LogP | 1.290 (est) |
| CAS DataBase Reference | 486-70-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2H-quinolizine-1-methanol, octahydro-, (1r-trans)-(486-70-4) |
Description and Uses
This lupin alkaloid was first obtained by Baumert and is present in numerous
plants of the Lupinus family, the most important sources being L. luteus, L.
niger and L. palmeri Wats. It crystallizes from light petroleum in colourless
rhombs and may be boiled without decomposition in a stream of hydrogen. It is
laevorotatory with [α]17D - 20.35° (EtOH) and is soluble in H20 and most
organic solvents but only sparingly so in petroleum ether. It is a sufficiently
strong base to liberate ammonia from its salts. The hydrochloride forms colourless rhombic crystals from aqueous EtOH, m.p. 2l2-3°C; [α]D - 14° (H20);
the hydriodide has m.p. 140-1 DC; aurichloride, m.p. 211-3°C; platinichloride
as yellow crystals, m.p. 166-166.5°C; (+)-tartrate, m.p. 171°C; [α]D + 15.5°
(EtOH); methochloride, m.p. 212-3°C; methiodide, m.p. 295-6°C; phenylurethane, m.p. 98-9°C; (-)-camphorsulphonate, m.p. 184°C; [α]D - 15.3° and the
(+)-camphorsulphonate, m.p. 182-3°C; [α]D + 22.5°. The formation of a
benzoyl derivative, m.p. 49-S00 C, oxidation of the alkaloid to lupininic acid,
C9H16.COOH, m.p. 255°C and dehydration to anhydrolupinine, ClOH17N, a
colourless oil of unpleasant odour, b.p. 216-7°C/726 mm all show the presence
of a primary alcoholic hydroxyl group. Lupinine does not contain a methylimino
group and behaves as a tertiary base. On exhaustive methylation it gives, in three
stages, trimethylamine and an unsaturated alcohol. From this, it may be deduced
that the alkaloid contains a bicyc1ic system. Several unsuccessful attempts were made to synthesize the alkaloid before this was finally achieved by Clemo and
his co-workers.
This alkaloid, like the others of the lupinane group, is of little importance in
medicine although the p-aminobenzoate has been shown to possess a marked
local anaesthetic action.
The (+ )-form of the alkaloid has m.p. 68°C; [α]D + 19.9° and yields the (-)-
tartrate, m.p.167-8°C; [α]D -15.8°.
The ι-form of lupinine occurs in seeds andherb of Lupinus luteous L., Chenopodiaceae,and other lupinus species. Its clinical applications are very limited.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H312-H332 |
| Precautionary statements | P261-P280h-P301+P312a-P304+P340-P321-P501a |
| Hazard Codes | Xi |
| Risk Statements | 20/21/22-41 |
| Safety Statements | 22-36/37-39-26 |
| RIDADR | 1544 |
| RTECS | OK5802000 |
| Hazard Note | Irritant |
| HazardClass | 6.1 |
| PackingGroup | III |