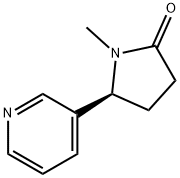
(S)-1-Methyl-5-(pyridin-3-yl)pyrrolidin-2-one , 98% , 486-56-6
Synonym(s):
(−)-Cotinine;(S)-1-Methyl-5-(3-pyridyl)-2-pyrrolidinone;S(−)-1-Methyl-5-(3-pyridyl)-2-pyrrolidone
CAS NO.:486-56-6
Empirical Formula: C10H12N2O
Molecular Weight: 176.22
MDL number: MFCD00077696
EINECS: 207-634-9
| Pack Size | Price | Stock | Quantity |
| 100mg | RMB292.80 | In Stock |
|
| 250mg | RMB548.00 | In Stock |
|
| 1g | RMB1632.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 40-42 °C(lit.) |
| Boiling point: | 250 °C150 mm Hg(lit.) |
| alpha | [α]D20 -18~-22° (c=1, C2H5OH) |
| Density | 1.1102 (rough estimate) |
| refractive index | 1.7110 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Sparingly) |
| form | Solid |
| pka | 4.72±0.12(Predicted) |
| color | Colourless to Very Dark Orange Oil |
| optical activity | [α]/D -21±2°, c = 1 in ethanol |
| Water Solubility | Not miscible or difficult to mix in water. Soluble in dimethyl sulfoxide (100 mM), ethanol (50 mg/ml, yielding a clear, faint yellow to yellow solution), methanol and chloroform. |
| Merck | 14,2553 |
| BRN | 83099 |
| Stability: | Stable. Incompatible with strong oxidizing agents. May be heat sensitive - store cold. |
| EPA Substance Registry System | Cotinine (486-56-6) |
Description and Uses
Cotinine is the major metabolite of nicotine. In the liver,
nicotine is rapidly metabolized to cotinine (70–80%) by
CYP2A6 and to nornicotine (5%) by CYP2A6 and CYP2B6.
With a half-life about 10-fold longer than that of nicotine
(15–19 h for cotinine versus 2–3 h for nicotine), cotinine
induces plasma concentrations of 1–3 mM in smokers. After
administration to rats, cotinine levels in the brain reach
fourfold those of nicotine at 4 h following injection. Cotinine
is not biotransformed in the brain, allowing accumulation
of this substance to levels greater than that of
nicotine.
Like nicotine, cotinine is able to induce dopamine release in
smokers and in superfused rat striatal slices in a dose- and
calcium-dependent manner via the nicotinic receptors, but
only at concentrations higher than those normally seen in
smokers. Indeed, administration of cotinine to smokers at
levels 10-fold that is seen following smoking had no observable
effect, suggesting that cotinine is not neuroactive at doses
found in smokers. However, cotinine also acts as an inhibitor
for nicotine binding in rat brain via desensitization of the
nicotinic receptor.
(-)-Cotinine is used to activate a subpopulation of α3/ α6β2 nAChRs in monkey striatum. It binds nicotinic- and muscarinic-type acetylcholine receptors with minimal receptor desensitization and demonstrates antipsychotic drug-like properties in behavioral models, neuroprotective properties in neurodegenerative disease models, and enhances attention in a delayed matching-to-sample task.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P301+P312+P330-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn,Xi,T,F |
| Risk Statements | 22-36/37/38-39/23/24/25-23/24/25-11 |
| Safety Statements | 7-16-36/37-45-36-26 |
| RIDADR | UN 1230 3/PG 2 |
| WGK Germany | 3 |
| RTECS | GN1925500 |
| F | 10 |
| HS Code | 29333990 |
| Hazardous Substances Data | 486-56-6(Hazardous Substances Data) |