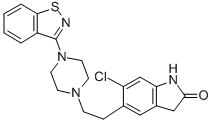
Ziprasidone HCl , ≥98% , 122883-93-6
CAS NO.:122883-93-6
Empirical Formula: C21H21ClN4OS
Molecular Weight: 412.943
MDL number: MFCD06795476
EINECS: 602-903-0
| Pack Size | Price | Stock | Quantity |
| 50mg | RMB55.20 | In Stock |
|
| 250MG | RMB175.20 | In Stock |
|
| 1G | RMB479.20 | In Stock |
|
| 5g | RMB1839.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >300 °C |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO > 10 mM |
| form | Powder |
| Stability: | Hygroscopic |
Description and Uses
Both p.o. and i.m. formulations of ziprasidone were launched in Sweden for the treatment of schizophrenia and agitated psychoses. It is the sixth marketed atypical antipsychotic after clozapine, risperidone, olanzapine, sertindole and quetiapine. The synthesis of ziprasidone involves a novel one-step process for the preparation of 3-(1- piperazinyl)-1,2-benzisothiazole followed by coupling with a chlorooxindole fragment. Ziprasidone is a very potent 5-HT2A/D2 antagonist with a ratio of about 11 in favor of the serotonin receptor. It also shows very high 5-HT2c antagonistic activity, high 5-HT1A agonistic and 5-HT1D antagonistic activity, as well as moderate antagonism of α1 and H1 receptors and moderate norepinephrine and serotonin reuptake inhibition. Its complex binding profile for serotonin and dopamine receptors resulted during clinical trials in high antipsychotic efficacy with low extrapyramidal side effects and also in antidepressive action with low propensity for weight gain in opposition to other atypical and typical neuroleptics. An intramuscular formulation of ziprasidone was demonstrated to be superior to haloperidol, a conventional neuroleptic, for the short-term treatment of agitation in acutely psychotic patients. When administered orally in the fed state, this well-tolerated agent which strongly binds to plasma proteins shows a bioavailability of about 60% which is almost 2 fold greater than in the fasted state. It is transformed into 4 circulating major metabolites by different enzyme systems. The small QTc prolongation observed with ziprasidone was found to be comparable to other antipsychotic drugs and it is considered to be without significant risk.
Ziprasidone (Geodon, Zeldox) was the fifth atypical antipsychotic to gain FDA approval. In the United States, Ziprasidone is approved for the treatment of schizophrenia, and the intramuscular injection form of ziprasidone is approved for acute agitation i