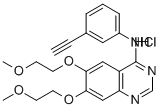
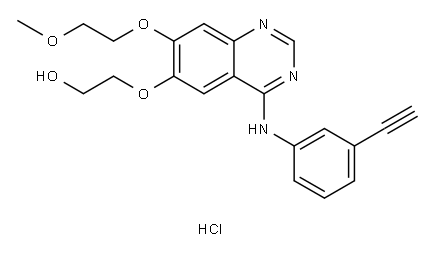
Erlotinib HCl (OSI-744) , ≥99% , 183319-69-9
CAS NO.:183319-69-9
Empirical Formula: C22H24ClN3O4
Molecular Weight: 429.9
MDL number: MFCD07781272
EINECS: 620-491-0
| Pack Size | Price | Stock | Quantity |
| 100MG | RMB23.20 | In Stock |
|
| 500MG | RMB31.20 | In Stock |
|
| 1g | RMB41.60 | In Stock |
|
| 5g | RMB124.00 | In Stock |
|
| 25g | RMB587.20 | In Stock |
|
| 100g | RMB2215.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 223-225°C |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 18 mg/ml with warming). |
| form | Yellow powder. |
| pka | pKa (25°): 5.42 |
| color | White or off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| InChI | InChI=1S/C22H23N3O4.ClH/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22;/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25);1H |
| InChIKey | GTTBEUCJPZQMDZ-UHFFFAOYSA-N |
| SMILES | C12C=C(OCCOC)C(OCCOC)=CC=1N=CN=C2NC1=CC=CC(=C1)C#C.Cl |
| CAS DataBase Reference | 183319-69-9(CAS DataBase Reference) |
Description and Uses
Erlotinib, launched as once daily oral treatment for patients with non-small-cell lung cancer (NSCLC), is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, and it is the second small-molecule drug to be marketed with this mechanism of action. Both erlotinib and its predecessor, gefitinib, are members of the anilinoquinazoline class of tyrosine kinase inhibitors. They compete with the binding of ATP to the intracellular tyrosine kinase domain of EGFR, thereby inhibiting receptor autophosphorylation and blocking downstream signal transduction. Erlotinib is prepared by the condensation of 3-ethynylaniline with 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline, which is a key intermediate obtained in five synthetic steps starting from ethyl 3,4- dihydroxybenzoate. In vitro, Erlotinib inhibits purified human EGFR tyrosine kinase with an IC50 of 2 nM and blocks EGFR autophosphorylation in cellular assays with an IC50 of 20nM. Treatment of human colon cancer cells with erlotinib was associated with growth inhibition, G1 cell cycle arrest, and apoptosis. Oral administration of erlotinib in athymic mice produced potent antitumor effects with an ED50 of 9.2 mg/kg/day for HN5 head and neck xenografts and 14 mg/ kg/day for A431 epidermoid xenografts. The absorption of Erlotinib following oral dosing is approximately 60%. Food greatly enhances the absorption allowing for almost 100% bioavailability of the dose. The time to reach peak plasma levels of the drug is about 4 hours, and the half-life is approximately 36 hours. Steady-state drug levels are reached in 7 to 8 days. Erlotinib has high protein binding (93%) and has an apparent volume of distribution of 232 L. It is metabolized primarily by CYP3A4 and to a lesser extent by CYP1A2 and CYP1A1. The drug is mainly excreted in the feces with less than 9% of the dose found in the urine. Erlotinib is labeled for the treatment of patients with locally advanced or metastatic NSCLC who have failed one or more previous chemotherapy regimens. The recommended dosage is 150 mg daily until disease progression is detected. In a randomized, double blind, placebo-controlled trial involving 731 patients, 150 mg/day oral dose of erlotinib resulted in a median overall survival of 6.7 months compared with 4.7 months in the placebo group (p<0.001). Progression-free survival was 9.9 weeks and 7.9 weeks in the erlotinib and placebo groups, respectively (p<0.001). Survival at one year was 31.2% in the erlotinib group versus 21.5% in the placebo group. The use of erlotinib showed greater benefit in patients with EGFR positive tumors and in those who never smoked. The most common adverse events reported in clinical trials were rash (9%) and diarrhea (6%). Elevations in liver function tests were also seen; however, these effects were mainly transient or associated with liver metastases. As previously noted for gefitinib, erlotinib is also shown to lack any clinical benefit in concurrent administration with platinum-based chemotherapy.
Erlotinib hydrochloride (V), a quinazoline derived small molecule inhibitor of epidermal growth factor receptor (EDGFR) tyrosine kinase, was approved in November, 2004, for the treatment of advanced or metastatic non-smallcell lung cancer. It belongs to the same class as gefitinib,another quinazoline approved for treatment of advanced lung cancer, but with improved pharmacokinetic properties. The molecule was originated by Pfizer and development initiated in collaboration with OSI, which assumed full rights to the drug when Pfizer merged with Warner Lambert. Subsequently, Genentech/Roche went into licensing agreement with OSI to develop and market the drug in the US and Worldwide.