Calcium phosphate , Biomedical grade, ≥98%, α-pHASEBASIS, <10μm , 7758-87-4
Synonym(s):
Calcium phosphate;β-TCP;β-Calcium phosphate tribasic;β-Tricalcium phosphate;tert-Calcium phosphate
CAS NO.:7758-87-4
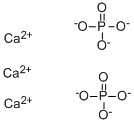
Empirical Formula: Ca3O8P2
Molecular Weight: 310.18
MDL number: MFCD00015984
EINECS: 231-840-8
| Pack Size | Price | Stock | Quantity |
| 5G | RMB671.20 | In Stock |
|
| 25G | RMB2687.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1670°C |
| Density | 3.14 |
| refractive index | 1.63 |
| FEMA | 3081 | TRICALCIUM PHOSPHATE |
| storage temp. | room temp |
| solubility | Practically insoluble in water. It dissolves in dilute hydrochloric acid and in dilute nitric acid. |
| form | aqueous suspension |
| color | White |
| PH | 6-8 (50g/l, H2O, 20°C) suspension |
| Odor | Odorless |
| Water Solubility | 0.1 g/L (25 ºC) |
| Merck | 13,1699 |
| Solubility Product Constant (Ksp) | pKsp: 28.68 |
| InChIKey | QORWJWZARLRLPR-UHFFFAOYSA-H |
| LogP | -2.15 |
| CAS DataBase Reference | 7758-87-4(CAS DataBase Reference) |
| EPA Substance Registry System | Tricalcium phosphate (7758-87-4) |
Description and Uses
Calcium Phosphate is the calcium salt of phosphoric acid with widely used applications. It occur abundantly in nature in several forms and are the principal minerals for the production of phosphate fertilizers and for a range of phosphorus compounds. For example, the tribasic variety (precipitated calcium phosphate), Ca3(PO4)2, is the principal inorganic constituent of bone ash. The acid salt Ca(H2PO4)2, produced by treating mineral phosphates with sulfuric acid, is employed as a plant food and stabilizer for plastics. It is a natural constituent of mammals, and it is a component of bone replacement transplants in much higher amounts with no toxicological problems.
Calcium phosphates are the largest group of artificial bone graft substitutes. This is mainly due to their close resemblance to the mineral components of bone. This Product can be used as a countermeasure for exposure to strontium and radium radionuclides. Upon oral uptake, calcium phosphate competes for and blocks the absorption of radium (Ra-226) and strontium (Sr-90) in the gastrointestinal (GI) tract.
manufacture of fertilizers, H3PO4 and P Compounds; manufacture of milk-glass, polishing and dental powders, porcelains, pottery; enameling; clarifying sugar syrups; in animal feeds; as noncaking agent; in the textile industry.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P271-P280 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36-24/25-22 |
| WGK Germany | 3 |
| HS Code | 2835 26 00 |
| Hazardous Substances Data | 7758-87-4(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rabbit > 2000 mg/kg |