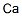Calcium , Null
Synonym(s):
Atomic calcium;CA006101;Calcium atom;Calcium element
| Pack Size | Price | Stock | Quantity |
| 50ml | RMB1287.20 | In Stock |
|
| 100ml | RMB2027.20 | In Stock |
|
| 500ml | RMB4908.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| form | Liquid |
| color | Clear colorless |
| Water Solubility | Miscible with water. |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Dielectric constant | 3.0(Ambient) |
Description and Uses
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic weight of 40.078 g/mol. Calcium is the fifth most abundant dissolved ion in seawater by both molarity and mass, after Na+, Cl-, Mg2+ and SO42-. Calcium metal is quite reactive. It readily forms a white coating of calcium nitride (Ca3N2) in air at room temperature. It reacts with water and the metal burns in air with an orangered flame, forming largely the nitride, but some oxide. In the visible portion of the spectrum of many stars, including the Sun, strong absorption lines of singly ionized calcium ions are evident. Prominent among these are the H line at 3968.5 A ° and the K line at 3933.7 A°of singly ionized calcium, or Ca II. For the Sun, and stars with low temperatures, the prominence of the H and K lines is an indication of strong magnetic activity in the chromosphere. Measurement of periodic variations of these active regions can also be used to deduce the rotational periods of these stars.
Calcium, plasma standard solution is used as a standard solution in analytical chemistry and atomic absorption spectroscopy. It is also used as a single-element standard solution for plasma emission spectrometry.