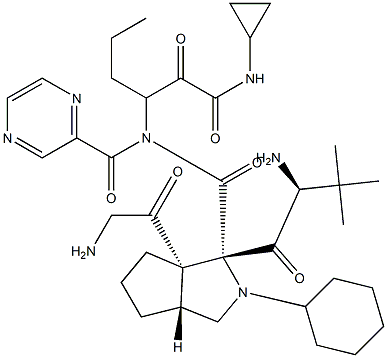
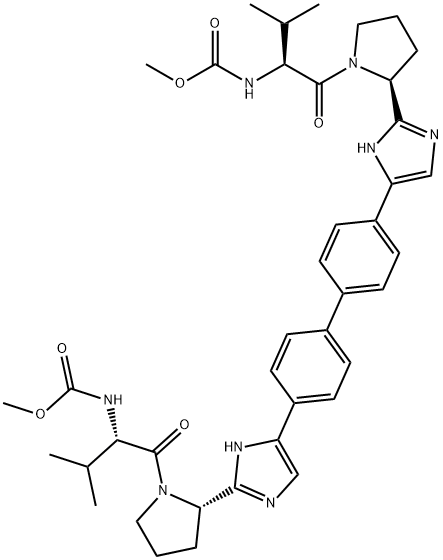
Telaprevir , ≥98% , 402957-28-2
Synonym(s):
2-(2-(2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-(1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide;LY-570310;VX-950
CAS NO.:402957-28-2
Empirical Formula: C36H53N7O6
Molecular Weight: 679.85
MDL number: MFCD11616089
EINECS: 609-814-6
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB319.20 | In Stock |
|
| 10MG | RMB623.20 | In Stock |
|
| 50MG | RMB2159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 116-123°C |
| Density | 1.25 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform, Methanol (Slightly) |
| pka | 11.84±0.20(Predicted) |
| form | Solid |
| color | Pale Yellow to Light Yellow |
Description and Uses
The hepatitis C virus (HCV) protease inhibitor telaprevir (VX-950, MP- 424,LY-570310) was approved by the U.S. FDA in May 2011 for the treatment of genotype 1 chronic HCV infection in adult patients in combination with peginterferon alfa and ribavirin (PR). Telaprevir and boceprevir (vide supra) are the first two HCV protease inhibitors to be approved for treatment of HCV infection. Telaprevir is a HCV NS3-4A protease inhibitor that exerts its antiviral effect by blocking the release of nonstructural viral proteins from a polyprotein precursor. Telaprevir is a potent inhibitor of the protease (IC50=10 nM) and is active in cell culture (HCV 1b replicon assay, EC50=354 nM). Telaprevir was identified from efforts to truncate a decamer peptide inhibitor derived from the natural substrate NS5A-5B and was guided by structure-based design. The ketoamide group of telaprevir forms a covalent, reversible bond with the active site serine hydroxyl of the protease and compensates for the loss of affinity resulting from truncation of the peptide. Despite the presence of the reactive keto-amide group, telaprevir is >500-fold less potent against other serine proteases. Synthesis of the key octahydrocyclopenta[c]pyrrole-1-carboxylic acid fragment of telaprevir is achieved by a-deprotonation of Boc-protected 3-azabicyclo[3.3.0]nonane followed by reaction with CO2 and resolution of the racemic acid. Alternatively, deprotonation is carried out in the presence of a chiral amine to give the enantiomerically enriched acid.
Labeled Telaprevir, intended for use as an internal standard for the quantification of Telaprevir by GC- or LC-mass spectrometry.
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Warning |
| Hazard statements | H361fd |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Safety Statements | 24/25 |
| HS Code | 29339900 |
| Hazardous Substances Data | 402957-28-2(Hazardous Substances Data) |