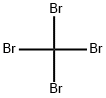
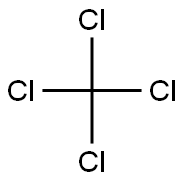
Tetrabromomethane , 98% , 558-13-4
Synonym(s):
Carbon tetrabromide
CAS NO.:558-13-4
Empirical Formula: CBr4
Molecular Weight: 331.63
MDL number: MFCD00000117
EINECS: 209-189-6
PRODUCT Properties
| Melting point: | 88-90 °C(lit.) |
| Boiling point: | 190 °C(lit.) |
| Density | 3,42 g/cm3 |
| vapor pressure | 40 mm Hg ( 96 °C) |
| refractive index | 1.5942 |
| Flash point: | 190°C |
| storage temp. | Store below +30°C. |
| solubility | soluble in Chloroform |
| form | Crystalline Solid |
| color | White to off-white |
| Water Solubility | insoluble |
| BRN | 1732799 |
| Dielectric constant | 7(22.0℃) |
| Exposure limits | ACGIH: TWA 0.1 ppm; STEL 0.3 ppm NIOSH: TWA 0.1 ppm(1.4 mg/m3); STEL 0.3 ppm(4 mg/m3) |
| CAS DataBase Reference | 558-13-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbon tetrabromide(558-13-4) |
| EPA Substance Registry System | Carbon tetrabromide (558-13-4) |
Description and Uses
Carbon tetrabromide is considered a highly toxic chemical, may be fatal if inhaled, swallowed, or absorbed through skin. It is metabolized in vitro to produce carbon monoxide but the in vivo significance has not been established. Under anaerobic reducing conditions it forms complexes with ferrous cytochrome P450. Carbon monoxide is detected as a metabolic product of the interaction. Carbon tetrabromide’s production and use in organic syntheses may result in its release to the environment through various waste streams. Carbon tetrabromide has been isolated from red algae, Asparagopsis toxiformis, found in the ocean near Hawaii. It was detected in water from treated chlorinated seawater used for drinking at oil platforms. Occupational exposure to carbon tetrabromide may occur through inhalation and dermal contact with this compound at workplaces where it is produced or used. The general population may be exposed to carbon tetrabromide via ingestion of drinking water. Acute exposures to high concentrations may cause upper respiratory tract irritation and injury to lungs, liver (hepatotoxicity) and kidneys (nephrotoxicity). Chronic exposure effects at very low levels will be almost entirely limited to liver injury. It is a potent lachrymator even at low exposure concentrations. Although carbon tetrabromide may release bromine ions during metabolism, clinical bromism is not expected to occur.
Carbon tetrabromide is used to a limited extent as a chemical intermediate. It has been isolated from red algae, Asparagopsis toxiformis, found in the ocean near Hawaii.
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302-H315-H318-H335 |
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xi,T+,N,Xn |
| Risk Statements | 37/38-41-36-26-52/53-22 |
| Safety Statements | 26-36-45-27-24-61 |
| RIDADR | UN 2516 6.1/PG 3 |
| OEB | B |
| OEL | TWA: 0.1 ppm (1.4 mg/m3), STEL: 0.3 ppm (4 mg/m3) |
| WGK Germany | 3 |
| RTECS | FG4725000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29033036 |
| Hazardous Substances Data | 558-13-4(Hazardous Substances Data) |