Titanium tetrachloride , 99.9%metalsbasis , 7550-45-0
Synonym(s):
Titanium tetrachloride;Titanium(IV) chloride;TTC
CAS NO.:7550-45-0
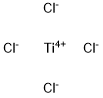
Empirical Formula: Cl4Ti
Molecular Weight: 189.68
MDL number: MFCD00011267
EINECS: 231-441-9
PRODUCT Properties
| Melting point: | −25 °C(lit.) |
| Boiling point: | 135-136 °C(lit.) |
| Density | 1.73 g/mL at 20 °C(lit.) |
| vapor pressure | 50 mm Hg ( 55 °C) |
| refractive index | 1.61 |
| Flash point: | 46 °F |
| storage temp. | Flammables area |
| solubility | H2O: soluble |
| form | Solution |
| color | Light yellow to dark brown |
| Specific Gravity | 1.726 |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9478 |
| Exposure limits | ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Dielectric constant | 40.0(Ambient) |
| Stability: | Stable. Reacts with water. Incompatible with moisture, ammonia, amines, alcohols, potassium and other chemically active metals. |
| CAS DataBase Reference | 7550-45-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Titanium tetrachloride(7550-45-0) |
| EPA Substance Registry System | Titanium tetrachloride (7550-45-0) |
Description and Uses
Titanium ore was first discovered in 1791 in Cornish beach
sands by an English clergyman, William Gregor. The actual
identification of the oxide was made a few years later by
a German chemist, M.H. Klaproth, who gave the metal
constituent of this oxide the name titanium, after the Titans of
Greek mythology. Pure metallic titanium was first produced in
the early 1900s in 1910 by M.A. Hunter at Rensselaer Polytechnic
Institute in cooperation with General Electric
Company.
Titanium tetrachloride is an inorganic compound that is an
important intermediate in the production of titanium metal
and the pigment titanium dioxide. On contact with humid air,
it forms opaque clouds of titanium dioxide (TiO2) and
hydrogen chloride (HCl). Early attempts to isolate titanium
metal from titanium tetrachloride were unsuccessful. The
process was improved and commercialized by William Kroll of
Luxembourg in the 1930s which involved the reduction of
titanium tetrachloride with magnesium in an inert gas atmosphere.
This process remains essentially unchanged today. The
primary use of titanium tetrachloride is for titanium dioxide
used in paints.
The production of titanium metal accounts for only 5% of
annual titanium mineral consumption, with the remainder
being used in the titanium pigment industry. Pigments are
produced using either a sulfate process or a more environmentally
acceptable carbochlorination process that converts
TiO2 into TiCl4. The latter process also supplies the TiCl4
necessary for the production of titanium metal.
Titanium (IV) tetrachloride (TiCl4) produces a dense white smoke-like vapor when exposed to moist air. It is used as smoke screens and for skywriting, as well in theatrical productions where fog or smoke is required for the scene.
Safety
| Symbol(GHS) |   GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H314-H330-H335 |
| Precautionary statements | P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338+P310 |
| Hazard Codes | C,F,Xi,T |
| Risk Statements | 36/37/38-67-65-63-48/20-34-14-11-23-40-37-48/23-39/23-20/21/22-36/38 |
| Safety Statements | 26-7/8-62-46-45-36/37/39-24/25-23-16-60 |
| RIDADR | UN 3289 6.1/PG 2 |
| WGK Germany | 2 |
| RTECS | XR1925000 |
| F | 21 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28273990 |
| Hazardous Substances Data | 7550-45-0(Hazardous Substances Data) |
| Toxicity | Not found naturally in the environment. Manufactured from titanium-containing minerals and is used to make metallic titanium, titanium dioxide, and other titanium compounds. An irritant to skin, eyes, mucus membranes, and lungs due to its interaction with water to form hydrochloric acid, excessive exposure can result in chemical bronchitus, pneumonia, and death. Severe burns may result from contact with liquid titanium tetrachloride. Although long term, high dose studies caused lung tumors in rodents, IARC and other agencies have not classified titanium tetrachloride for its potential as a human carcinogen. |