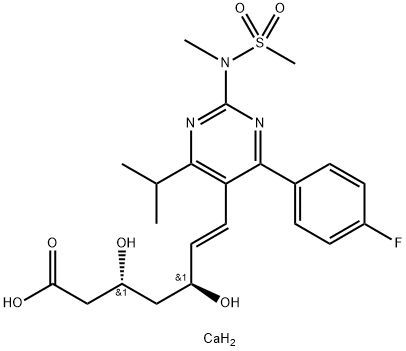
PitavastatinCalcium , 10mMinDMSO , 147526-32-7
CAS NO.:147526-32-7
Empirical Formula: C25H26CaFNO4
Molecular Weight: 463.56
MDL number: MFCD01937979
EINECS: 807-641-2
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB559.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >138oC (dec.) |
| alpha | D20 +23.1° (c = 1.00 in acetonitrile/water) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| optical activity | [α]/D +19 to +24°, c =1 in acetonitrile: water (1:1) |
| Stability: | Hygroscopic |
| InChIKey | ADXNRJCYUMOXPZ-MNGIKBOWNA-N |
| SMILES | C(/C1C(C2CC2)=NC2C=CC=CC=2C=1C1C=CC(F)=CC=1)=C\[C@@H](O)C[C@@H](O)CC(=O)O.[Ca] |&1:22,25,r| |
| CAS DataBase Reference | 147526-32-7(CAS DataBase Reference) |
Description and Uses
Pitavastatin, launched for the treatment of hypercholesterolemia, belongs to the family of second-generation statins, also referred to as superstatins due to their improved efficacy as cholesterol lowering agents. Like other statins, pitavastatin reduces plasma cholesterol levels by competitively inhibiting HMG-CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis in the liver. It is a more potent inhibitor of HMG-CoA reductase than the previously marketed statins and has the potential benefit of not undergoing significant metabolism by CYP3A4. Pitavastatin is synthesized in a multi-step sequence, including the key step of introducing the dihydroxyheptenoate side chain by cross-coupling of a 3-iodoquinoline intermediate with an alkenylborane reagent. Unlike rosuvastatin, pitavastatin has a high oral bioavailability (~80%). Plasma protein binding is also high for pitavastatin (>95%), and regardless of the dosing, the highest tissue levels are found in the liver, its target organ. After oral administration, the peak plasma concentration is reached at ,0.8 h and the mean elimination half-life is ~11 h. Pitavastatin is only minimally metabolized, mainly by CYP2C8 and CYP2C9, and the predominant route of elimination of the parent drug and its metabolites is by means of bile excretion followed by elimination in the feces. In clinical studies, oral doses of 2–4 mg/day of pitavastatin produced dose-dependent reduction in LDL-cholesterol levels by 40–48% from baseline in patients with heterozygous familial hypercholesterolemia. In a 12-week double-blind comparative study, pitavastatin (2 mg/day) was more effective than pravastatin (10 mg/day) in reducing LDL-cholesterol levels (38 and 18%, respectively); however, both agents produced similar increases in HDL-cholesterol (~9%). The drug was well tolerated and the adverse reactions were mild and transient.
A competitive inhibitor of HMG-CoA reductase. Antilipemic.
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H301+H311+H331-H315-H319 |
| Precautionary statements | P501-P261-P270-P271-P264-P280-P337+P313-P305+P351+P338-P361+P364-P332+P313-P301+P310+P330-P302+P352+P312-P304+P340+P311-P403+P233-P405 |
| Safety Statements | 24/25 |
| HS Code | 29339900 |
| Toxicity | LDLo orl-rat: 500 mg/kg OYYAA2 56,67,1998 |

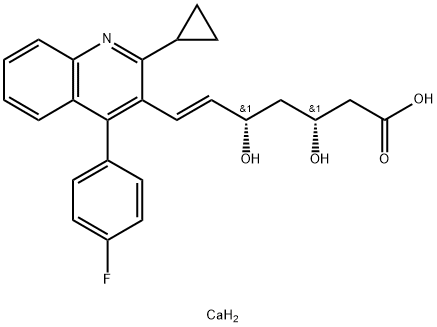
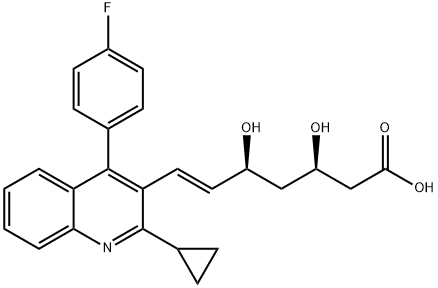
![t-Butyl(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-isopropylidenedioxy-6-heptenoate](https://img.chemicalbook.com/CAS/GIF/147489-06-3.gif)