Efavirenz , 10mMinDMSO , 154598-52-4
Synonym(s):
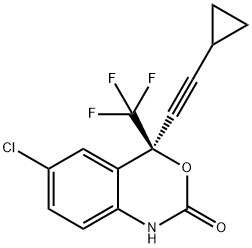
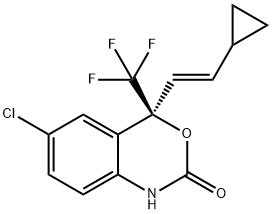
(4S)-6-Chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one;(4S)-6-Chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one;Aryl halide chemistry informer library compound X17;Efavirenz
CAS NO.:154598-52-4
Empirical Formula: C14H9ClF3NO2
Molecular Weight: 315.67
MDL number: MFCD05662344
EINECS: 620-492-6
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 139-141°C |
| Boiling point: | 340.6±42.0 °C(Predicted) |
| alpha | D20 -84.7° (c = 0.005 g/ml in CH3Cl); D25 -94.1° (c = 0.300 in methanol) |
| Density | 1.53±0.1 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| pka | 10.2(at 25℃) |
| form | powder or crystals |
| color | white to beige |
| optical activity | [α]/D -90 to -100°, c = 1 in methanol |
| Water Solubility | 8mg/L(temperature not stated) |
| λmax | 247nm(MeOH)(lit.) |
| Merck | 14,3521 |
| BCS Class | 4 |
| EPA Substance Registry System | 2H-3,1-Benzoxazin-2-one, 6-chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4S)- (154598-52-4) |
Description and Uses
Efavirenz D5 was launched as Sustiva in the US for the treatment of infection by HIV, the virus causing AIDS, in combination with other anti-retroviral agents.
Efavirenz D5 is a non-nucleoside reverse transcriptase inhibitor (NNRTI) belonging to the 3,1-benzoxazin-2-one chemical class. It is the third non-nucleoside reverse transcriptase inhibitor to have been launched to date, after Nevirapine (1996) and Delavirdine (1997), increasing the arsenal of anti-HIV drugs for treating infected patients in dual or triple combination with nucleoside or other non-nucleoside RTIs, or protease inhibitors.
Efavirenz D5 can be obtained by two related ways of six steps from 4-chloroaniline ; one of them is based on asymmetric synthesis by enantioselective addition of an acetylide to a trifluoroacetophenone. The anti-HIV activity of Efavirenz D5 was demonstrated against most wild-type and clinical strains of HIV-1, including those with the most frequently observed mutations. Efavirenz D5 has a better pharmacokinetic profile when compared with the preceding drugs of this class ; in particular, in a long-term experiment conducted in cynomolgus monkeys, Efavirenz D5 was shown to easily cross the blood brain barrier leading to an increase of the antiviral concentration in cerebrospinal fluid.
For use in combination treatment of HIV infection (AIDS)
Safety
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H302-H360-H410 |
| Precautionary statements | P202-P264-P270-P273-P301+P312-P308+P313 |
| Hazard Codes | N |
| Risk Statements | 50 |
| Safety Statements | 61 |
| RIDADR | UN3082 - class 9 - PG 3 - DOT NA1993 - Environmentally hazardous substances, liquid, n.o.s. HI: all (not BR) |
| WGK Germany | 3 |
| RTECS | DM3440000 |
| HS Code | 2934990002 |
| Hazardous Substances Data | 154598-52-4(Hazardous Substances Data) |