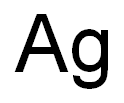Silver powder , 99.95%metalsbasis,≤10μm , 7440-22-4
Synonym(s):
Silver;Silver nanowire;Silverjet DGH-55HTG;Silverjet DGH-55LT-25C;Silverjet DGP-40LT-15C
CAS NO.:7440-22-4
Empirical Formula: Ag
Molecular Weight: 107.87
MDL number: MFCD00003397
EINECS: 231-131-3
| Pack Size | Price | Stock | Quantity |
| 1g | RMB67.20 | In Stock |
|
| 5G | RMB150.40 | In Stock |
|
| 25G | RMB500.00 | In Stock |
|
| 100G | RMB1746.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 960 °C(lit.) |
| Boiling point: | 2212 °C(lit.) |
| Density | 1.135 g/mL at 25 °C |
| vapor density | 5.8 (vs air) |
| vapor pressure | 0.05 ( 20 °C) |
| refractive index | n |
| Flash point: | 232 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | wool |
| color | Yellow |
| Specific Gravity | 10.49 |
| Odor | Odorless |
| PH | 0.5 (20°C in H2O) |
| Resistivity | 1-3 * 10^-5 Ω-cm (conductive paste) &_& 1.59 μΩ-cm, 20°C |
| Water Solubility | insoluble |
| Sensitive | Light Sensitive |
| Merck | 13,8577 |
| Exposure limits | TLV-TWA (metal dusts and fumes) 0.1
mg/m3 (ACGIH), 0.01 mg/m3 (MSHA and
OSHA), soluble compounds 0.01 mg/m3
(AIGIH). |
| Stability: | Stable. Substances to be avoided include strong acids and strong bases, tartaric acid, oxalic acid. Blackened by contact with ozone, hydrogen sulfide, sulfur. Powder is highly flammable. |
| CAS DataBase Reference | 7440-22-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Silver(7440-22-4) |
| EPA Substance Registry System | Silver (7440-22-4) |
Description and Uses
Silver is one of the earliest known metals. Silver has no known physiologic or biologic function, though colloidal silver is widely sold in health food stores. Silver has high thermal and electrical conductivity and resists oxidation in air that is devoid of hydrogen sulfide.
Silver has a multitude of uses and practical applications both in its elemental metallic formand as a part of its many compounds. Its excellent electrical conductivity makes it ideal for usein electronic products, such a computer components and high-quality electronic equipment.It would be an ideal metal for forming the wiring in homes and transmission lines, if it weremore abundant and less expensive.
Metallic silver has been used for centuries as a coinage metal in many countries. Theamount of silver now used to make coins in the United States has been reduced drastically byalloying other metals such as copper, zinc, and nickel with silver.
Silver is used as a catalyst to speed up chemical reactions, in water purification, and inspecial high-performance batteries (cells). Its high reflectivity makes it ideal as a reflectivecoating for mirrors.
Several of its compounds were not only useful but even essential for the predigital photographicindustry. Several of the silver salts, such as silver nitrate, silver bromide, and silverchloride, are sensitive to light and, thus, when mixed with a gel-type coating on photographicfilm or paper, can be used to form light images. Most of the silver used in the United Statesis used in photography.
Photochromic (transition) eyeglasses that darken as they are exposed to sunlight have asmall amount of silver chloride imbedded in the glass that forms a thin layer of metallic silverthat darkens the lens when struck by sunlight. This photosensitive chemical activity is thenreversed when the eyeglasses are removed from the light. This chemical reversal results from asmall amount of copper ions placed in the glass. This reaction is repeated each time the lensesare exposed to sunlight.
Safety
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | H302-H373-H410 |
| Precautionary statements | P273-P301+P312+P330-P314 |
| Hazard Codes | N,Xn,T,Xi,F |
| Risk Statements | 22-38-20/21-10-40-34-23/24/25-36/37/38-67-36-11-50/53-36/38 |
| Safety Statements | 26-24/25-25-45-36/37/39-23-16-7-61 |
| OEB | E |
| OEL | TWA: 0.01 mg/m3 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 3 |
| RTECS | VW3500000 |
| F | 8 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 71069110 |
| Hazardous Substances Data | 7440-22-4(Hazardous Substances Data) |
| Toxicity | PEL (OSHA) 0.01 mg/m3 TLV-TWA (ACGIH) 0.1 mg/m3 (silver metal) TLV-TWA (ACGIH) 0.01 mg/m3 (soluble silver compounds, as Ag) |
| IDLA | 10 mg Ag/m3 |