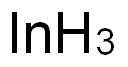Indium , 99.99%MetalsBasis, ≥200 , 7440-74-6
CAS NO.:7440-74-6
Empirical Formula: In
Molecular Weight: 114.82
MDL number: MFCD00134048
EINECS: 231-180-0
| Pack Size | Price | Stock | Quantity |
| 2g | RMB83.20 | In Stock |
|
| 5g | RMB183.20 | In Stock |
|
| 10G | RMB271.20 | In Stock |
|
| 25g | RMB583.20 | In Stock |
|
| 50G | RMB902.40 | In Stock |
|
| 100g | RMB1647.20 | In Stock |
|
| 250G | RMB3992.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 156.6 °C (lit.) |
| Boiling point: | 2000 °C |
| Density | 7.3 g/mL at 25 °C (lit.) |
| vapor pressure | <0.01 mm Hg ( 25 °C) |
| Flash point: | 2072°C |
| storage temp. | no restrictions. |
| solubility | soluble in acid solutions |
| form | wire |
| color | White |
| Specific Gravity | 7.31 |
| Flame Color | Indigo blue |
| Resistivity | 8.37 μΩ-cm |
| Water Solubility | insoluble |
| Merck | 14,4947 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents, sulfur. |
| LogP | 5.9 at 22℃ |
| CAS DataBase Reference | 7440-74-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Indium(7440-74-6) |
| EPA Substance Registry System | Indium (7440-74-6) |
Description and Uses
Indium lies in Group 13 (13th vertical column of the periodic table). it shows a wide variety of properties. It is considered to be metal of the 'poor metals' group.
Indium (symbol Ga; CAS# 7440-74-6) is widely used in the electronics industry, its radioisotopes are used for diagnostic imaging in medicine. Though this element is not essential for human nutrition, it is widely distributed in low concentrations in the environment (Smith et al., 1978).
Radioisotopes of indium have been used to label phagocytes and lymphocytes to localize inflammatory lesions (Dudley and Marrer, 1952; Abrams and Murrer, 1993). Despite early optimism, neither element has found wide use as a treatment for malignancies.
Indium’s low melting point is the major factor in determining its commercial importance.This factor makes it ideal for soldering the lead wires to semiconductors and transistors in theelectronics industry. The compounds of indium arsenide, indium antimonide, and indiumphosphide are used to construct semiconductors that have specialized functions in the electronicsindustry.
Another main use is as an alloy with other metals when it will lower the melting point ofthe metals with which it is alloyed. Alloys of indium and silver and indium and lead have theability to carry electricity better than pure silver and lead.
Indium is used as a coating for steel bearings to increase their resistance to wear. It alsohas the ability to “wet” glass, which makes it an excellent mirror surface that lasts longer than mercury mirrors. Sheets of indium foil are inserted into nuclear reactors to help control thenuclear fission reaction by absorbing some of the neutrons.
Safety
| Symbol(GHS) |  GHS09 |
| Signal word | Danger |
| Hazard statements | H411 |
| Precautionary statements | P273-P391-P501 |
| Hazard Codes | C,Xn,F,Xi |
| Risk Statements | 25-26-34-36/37/38-20/21/22-20-11-36/38 |
| Safety Statements | 9-16-36/37/39-36-26-45-28 |
| RIDADR | UN 3089 4.1/PG 2 |
| OEB | D |
| OEL | TWA: 0.1 mg/m3 [*Note: The REL also applies to other indium compounds (as In).] |
| WGK Germany | 3 |
| RTECS | NL1050000 |
| TSCA | Yes |
| HS Code | 8112 99 70 |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 7440-74-6(Hazardous Substances Data) |