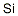Silicon Standard , SP , 7440-21-3
CAS NO.:7440-21-3
Empirical Formula: H4Si
Molecular Weight: 32.12
MDL number: MFCD00085311
EINECS: 231-130-8
PRODUCT Properties
| Melting point: | 1410 °C(lit.) |
| Boiling point: | 2355 °C(lit.) |
| Density | 2.33 g/mL at 25 °C(lit.) |
| bulk density | 0.07-0.08g/cm3 |
| storage temp. | Flammables area |
| solubility | insoluble in H2O, acid solutions; soluble in alkaline solutions |
| form | powder |
| Specific Gravity | 2.42 |
| color | White |
| PH | 13.5 (H2O, 20°C) |
| Odor | Odorless |
| Water Solubility | INSOLUBLE |
| semiconductor properties | <111>P-type |
| Crystal Structure | Cubic, Diamond Structure - Space Group Fd3m |
| Sensitive | Air Sensitive |
| Merck | 13,8565 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Dielectric constant | 2.4(Ambient) |
| Stability: | Stable. Fine powder is highly flammable. Incompatible with oxidizing agents, bases, carbonates, alkali metals, lead and aluminium oxides, halogens, carbides, formic acid. |
| InChIKey | BLRPTPMANUNPDV-UHFFFAOYSA-N |
| CAS DataBase Reference | 7440-21-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silicon(7440-21-3) |
| EPA Substance Registry System | Silicon (7440-21-3) |
Description and Uses
Silicon’s tetravalent pyramid crystalline structure, similar to tetravalent carbon, results ina great variety of compounds with many practical uses. Crystals of silicon that have beencontaminated with impurities (arsenic or boron) are used as semiconductors in the computerand electronics industries. Silicon semiconductors made possible the invention of transistorsat the Bell Labs in 1947. Transistors use layers of crystals that regulate the flow of electric current.Over the past half-century, transistors have replaced the vacuum tubes in radios, TVs,and other electronic equipment that reduces both the devices’ size and the heat produced bythe electronic devices.
Silicon can be used to make solar cells to provide electricity for light-activated calculatorsand satellites. It also has the ability to convert sunlight into electricity.When mixed with sodium carbonate (soda ash) and calcium carbonate (powdered limestone)and heated until the mixture melts, silica (sand) forms glass when cooled. Glass ofall types has near limitless uses. One example is Pyrex, which is a special heat-resistant glassthat is manufactured by adding boron oxide to the standard mixture of silica, soda ash, andlimestone. Special glass used to make eyewear adds potassium oxide to the above standardmixture.
Silicon is also useful as an alloy when mixed with iron, steel, copper, aluminum, andbronze. When combined with steel, it makes excellent springs for all types of uses, includingautomobiles.
When silicon is mixed with some organic compounds, long molecular chains known assilicone polymers are formed. By altering the types of organic substances to these long siliconepolymer molecules, a great variety of substances can be manufactured with varied physicalproperties. Silicones are produced in liquid, semisolid, and solid forms. Silicones may berubbery, elastic, slippery, soft, hard, or gel-like. Silicone in its various forms has many commercialand industrial uses. Some examples are surgical/reconstructive implants, toys, SillyPutty, lubricants, coatings, water repellents for clothing, adhesives, cosmetics, waxes, sealants,and electrical insulation.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Warning |
| Hazard statements | H228 |
| Precautionary statements | P210-P240-P241-P280-P370+P378 |
| Hazard Codes | T,F |
| Risk Statements | 11 |
| Safety Statements | 26-36/37-45-7/9-33-16-36 |
| RIDADR | UN 2922 8/PG 2 |
| OEB | B |
| OEL | TWA: 10 mg/m3 (total) |
| WGK Germany | 2 |
| RTECS | VW0400000 |
| Autoignition Temperature | 780°C |
| TSCA | Yes |
| HS Code | 3822 00 00 |
| HazardClass | 4.1 |
| PackingGroup | III |
| Hazardous Substances Data | 7440-21-3(Hazardous Substances Data) |