Probenecid , ≥98% , 57-66-9
Synonym(s):
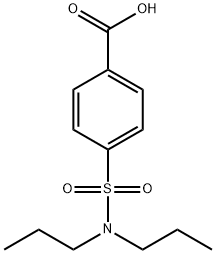
p-(Dipropylsulfamoyl)benzoic acid;Probenecid
CAS NO.:57-66-9
Empirical Formula: C13H19NO4S
Molecular Weight: 285.36
MDL number: MFCD00038402
EINECS: 200-344-3
| Pack Size | Price | Stock | Quantity |
| 5G | RMB43.20 | In Stock |
|
| 25G | RMB157.60 | In Stock |
|
| 100G | RMB513.60 | In Stock |
|
| 500g | RMB1839.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 194-196°C |
| Boiling point: | 438.0±47.0 °C(Predicted) |
| Density | 1.2483 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | Store at RT |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 5.8(at 25℃) |
| color | White to Off-White |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| Merck | 14,7754 |
| Stability: | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-66-9(CAS DataBase Reference) |
| NIST Chemistry Reference | P-(dipropylsulfamoyl) benzoic acid(57-66-9) |
| EPA Substance Registry System | Probenecid (57-66-9) |
Description and Uses
Probenecid is insoluble in water and acidic solutions but is soluble in alkaline solutions buffered to pH 7.4. Probenecid initially was synthesized as a result of studies in the 1940s on sulfonamides that indicated the sulfonamides decreased the renal clearance of penicillin, extending the half-life of penicillin as supplies diminished. Probenecid thus was initially used—and is still indicated—for that purpose. Probenecid promotes the excretion of uric acid by inhibiting the urate anion exchange transporter (URAT 1), decreasing the reabsorption of uric acid in the proximal tubules. The overall effect is to decrease plasma uric acid concentrations, thereby decreasing the rate and extent of urate crystal deposition in joints and synovial fluids. Within the series of N-dialkylsulfamyl benzoates from which probenecid is derived, renal clearance of these compounds is decreased as the length of the N-alkyl substituents is increased. Uricosuric activity increases with increasing size of the alkyl group in the series methyl, ethyl, and propyl.
It is a uricosuric drug, that is, it increases uric acid excretion in the urine. It is primarily used in treating gout and hyperuricemia. Probenecid was developed as an alternative to caronamide to competitively inhibit renal excretion of some drugs, thereby increasing their plasma concentration and prolonging their effects.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P301+P312+P330 |
| Hazard Codes | Xn |
| Risk Statements | 22-40 |
| Safety Statements | 36/37-24/25 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | DG9400000 |
| TSCA | Yes |
| HS Code | 29350090 |
| Hazardous Substances Data | 57-66-9(Hazardous Substances Data) |