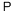Phosphorus solution , analyticalstandard,1000ug/mlinwater , 7723-14-0
Synonym(s):
P;Phosphorus;CAF;PCAF;Phosphorene
CAS NO.:7723-14-0
Empirical Formula: P
Molecular Weight: 30.97
MDL number: MFCD00133771
EINECS: 918-594-3
PRODUCT Properties
| Melting point: | 280 °C (white)(lit.) |
| Boiling point: | 280℃ |
| Density | 2.34 g/mL at 25 °C(lit.) |
| bulk density | 1100kg/m3 |
| vapor density | 0.02 (vs air) |
| vapor pressure | 0.03 mm Hg ( 21 °C) |
| Flash point: | 30°C |
| storage temp. | 2-8°C |
| solubility | insoluble |
| form | powder (red) |
| color | Red-brown |
| Specific Gravity | 2.34 |
| Odor | Acrid fumes when exposed to air |
| Flame Color | Pale blue-green |
| PH | 3 at 37℃ and 500-10000mg/L |
| Resistivity | 10 μΩ-cm, 20°C |
| Water Solubility | insoluble |
| Merck | 13,7433 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Dielectric constant | 4.1(34℃) |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents, strong bases. Light and heat sensitive. |
| CAS DataBase Reference | 7723-14-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphorus atom(7723-14-0) |
| EPA Substance Registry System | Phosphorus (7723-14-0) |
Description and Uses
White or yellow white phosphorus is a yellow waxy or colourless, transparent, volatile crystalline solid, waxy appearance with a garlic-like odour. On exposure to light, it darkens and ignites in air. It is also called yellow phosphorus colour because of impurities. White phosphorus does not occur naturally but is manufactured from phosphate rocks. It is insoluble in water, slightly soluble in benzene, ethanol, and chloroform, and is soluble in carbon disulphide. White phosphorus reacts rapidly with oxygen, easily catching fire at temperatures 10°C–15°C above room temperature. White phosphorus is used by the military in various types of ammunition and to produce smoke for concealing troop movements and identifying targets. It is also used by industry to produce phosphoric acid and other chemicals for use in fertilisers, food additives, and cleaning compounds. Small amounts of white phosphorus were used in the past in pesticides and fireworks.White phosphorus is used mainly for producing phosphoric acid and other chemicals. These chemicals are used to make fertilisers, additives in foods and drinks, cleaning compounds, and other products. In the military, white phosphorus is used in ammunitions such as mortar and artillery shells, and grenades.
Phosphorus is an essential constituent of plants and animals, being present in deoxyribonucleic acid (DNA), bones, teeth and other components of high biological importance. Phosphorus does not occur in its elemental state in nature, as it readily oxidises and therefore is deposited as phosphate rock. The remaining elements of group 15 are mostly obtained from minerals, but can also be found in their elemental form in the earth’s crust.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Warning |
| Hazard statements | H228-H412 |
| Precautionary statements | P210-P240-P241-P273-P280-P370+P378 |
| Hazard Codes | F,N,C,T+ |
| Risk Statements | 11-16-52/53-50-35-26/28-17 |
| Safety Statements | 7-43-61-43C-45-38-26-5-27-6 |
| RIDADR | UN 1338 4.1/PG 3 |
| OEB | D |
| OEL | TWA: 0.1 mg/m3 |
| WGK Germany | 2 |
| RTECS | TH3495000 |
| F | 10-21 |
| Autoignition Temperature | White phosphorus: 29 °C Red phosphorus: 260 °C |
| TSCA | Yes |
| HazardClass | 4.1 |
| PackingGroup | III |
| HS Code | 28047000 |
| Hazardous Substances Data | 7723-14-0(Hazardous Substances Data) |
| Toxicity | LD50 oral (rat) 3 mg/kg PEL (OSHA) 0.1 mg/m3 TLV-TWA (ACGIH) 0.02 ppm (0.1 mg/m3) |
| IDLA | 5 mg/m3 |