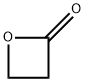
β-Propiolactone , >95.0%(GC) , 57-57-8
Synonym(s):
3-Hydroxypropionic acid lactone;Hydracrylic acid β-lactone;Oxetan-2-one
CAS NO.:57-57-8
Empirical Formula: C3H4O2
Molecular Weight: 72.06
MDL number: MFCD00005169
EINECS: 200-340-1
| Pack Size | Price | Stock | Quantity |
| 1ML | RMB175.20 | In Stock |
|
| 5ML | RMB606.40 | In Stock |
|
| 25ML | RMB2312.00 | In Stock |
|
| 100ML | RMB8799.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | -33 °C (lit.) |
| Boiling point: | 162 °C (lit.) |
| Density | 1.146 g/mL at 25 °C (lit.) |
| vapor pressure | 3 at 25 °C (NIOSH, 1997) |
| refractive index | n |
| Flash point: | 158 °F |
| storage temp. | -20°C |
| solubility | Miscible with acetone, alcohol, chloroform, and ether (Windholz et al., 1983) |
| form | Liquid |
| color | Colorless liquid with a sweet but irritating odor |
| Odor | pungent odor |
| Water Solubility | 37 g/100 mL |
| Sensitive | Moisture Sensitive |
| Merck | 14,7820 |
| Henry's Law Constant | 7.6 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Stability: | Moisture Sensitive, Very Hygroscopic |
| InChIKey | VEZXCJBBBCKRPI-UHFFFAOYSA-N |
| CAS DataBase Reference | 57-57-8(CAS DataBase Reference) |
| NIST Chemistry Reference | «beta»-Propiolactone(57-57-8) |
| IARC | 2B (Vol. 4, Sup 7, 71) 1999 |
| EPA Substance Registry System | beta-Propiolactone (57-57-8) |
Description and Uses
Beta-propiolactone is a colorless liquid with a strong, slightly
sweet odor. It may occur naturally, but no clear documentation
of its occurrence in nature was found, and it must be synthesized
for commercial purposes. Beta-propiolactone is unstable
at room temperature but stable when stored at 5 ℃ in glass
containers.
Its tendency to be unstable and react with other molecules in
the vicinity is responsible for both its toxicity and its usefulness.
Significant commercial production of beta-propiolactone took
place during the late 1950s through the mid-1970s, when it was
widely used in chemical synthesis in reactions with other
molecules to produce new chemicals. All lactones are characterized
by a ring structure consisting of two or more carbon
atoms – as can be seen from its structure, beta-propiolactone
has three in its ring – and a single oxygen, coupled with an
adjacent ketone. The fewer the carbons in the ring, the more
‘strained’ is the ring structure and the more unstable and reactive
its characteristics. When the ring bonds break, the betapropiolactone
molecules attach to other nearby molecules.
As late as 1974, b-propiolactone was used in the United States in the preparation of acrylic acid and acrylate esters. Today, its principal significance is as a reactive intermediate in organic syntheses; a small amount is treated with ammonia to provide balanine. b-Propiolactone was also used as a disinfectant. It appeared to be an attractive replacement for formaldehyde due to its 25-fold greater disinfecting power, but it has since been abandoned because of its carcinogenic properties.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H315-H319-H330-H350 |
| Precautionary statements | P202-P260-P264-P302+P352-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T+ |
| Risk Statements | 45-26-36/38 |
| Safety Statements | 53-45-99 |
| RIDADR | UN 3382 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | RQ7350000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 29322090 |
| Hazardous Substances Data | 57-57-8(Hazardous Substances Data) |
| Toxicity | LC50 (inhalation) for rats 25 ppm/6-h (quoted, RTECS, 1985). |