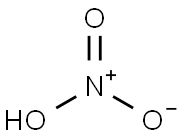
Nitric acid , GR,65-68% , 7697-37-2
Synonym(s):
Hydrogen Nitrate, Azotic Acid,Aqua Fortis, Aquafortis;Nitric acid solution
CAS NO.:7697-37-2
Empirical Formula: HNO3
Molecular Weight: 63.01
MDL number: MFCD00011349
EINECS: 231-714-2
PRODUCT Properties
| Melting point: | -42 °C |
| Boiling point: | 120.5 °C(lit.) |
| Density | 1.41 g/mL at 20 °C |
| vapor density | 1 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| Flash point: | 120.5°C |
| storage temp. | Store at +2°C to +25°C. |
| solubility | Miscible with water. |
| pka | -1.3(at 25℃) |
| form | Liquid, Double Sub-Boiling Quartz Distillation |
| Specific Gravity | d 20/4 1.4826 |
| color | colorless to deep yellow |
| PH Range | 1 |
| Odor | Suffocating fumes detectable at <5.0 ppm |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution); |
| Water Solubility | >100 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,6577 |
| Exposure limits | TLV-TWA 2 ppm (5 mg/m3) (ACGIH,
MSHA, OSHA, and NIOSH); STEL 4 ppm
(~10 mg/m3) (ACGIH). |
| Dielectric constant | 55.0(14℃) |
| LogP | -0.773 (est) |
| CAS DataBase Reference | 7697-37-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Nitric acid(7697-37-2) |
| EPA Substance Registry System | Nitric acid (7697-37-2) |
Description and Uses
Nitric acid is a colorless, corrosive liquid that is the most common nitrogen acid. It has been used for hundreds of years. Nitric acid is a mineral acid that was called spirit of nitre and aqua fortis, which means strong water.
Fuming nitric acid is named because of the fumes emitted by acid when it combines with moist air. Fuming nitric acid is highly concentrated and is labeled either red fuming nitric acid or white fuming nitric acid. Red fuming nitric acid, as the name implies, emits a reddishbrown fume on exposure to air. The color comes from nitrogen dioxide, which is liberated on exposure to air. The nitric acid concentration of red fuming nitric acid is approximately 85% or greater, with a substantial amount of dissolved nitrogen dioxide. White fuming nitric acid is highly concentrated anhydrous nitric acid with concentrations of 98–99%; the remaining 1–2% is water and nitrogen dioxide. Most commercial grade nitric acid has a concentration of between 50% and 70%.
Nitric acid is an important starting material for the production of fertilizers and chemicals. Diluted nitric acid is used for dissolving and etching metals Product Data Sheet
Safety
| Symbol(GHS) |    GHS03,GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H272-H290-H314-H331 |
| Precautionary statements | P210-P220-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | C,O,Xi,T+ |
| Risk Statements | 8-35-34-20-41-37/38-36/38-26/27 |
| Safety Statements | 23-26-36-45-36/37/39-39-60-28 |
| OEB | B |
| OEL | TWA: 2 ppm (5 mg/m3), STEL: 4 ppm (10 mg/m3) |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 1 |
| RTECS | QU5900000 |
| F | 8 |
| TSCA | Yes |
| HS Code | 2808 00 00 |
| HazardClass | 8 |
| PackingGroup | II |
| Hazardous Substances Data | 7697-37-2(Hazardous Substances Data) |
| Toxicity | LC50 inhal (rat) 2500 ppm (1 h) PEL (OSHA) 2 ppm (5 mg/m3) TLV-TWA (ACGIH) 2 ppm (5.2 mg/m3) STEL (ACGIH) 4 ppm (10 mg/m3) |
| IDLA | 25 ppm |