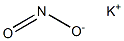Nitrite Standard , 1000ug/mlinwater , 7632-00-0
Synonym(s):
Sodium Nitrite;Sodium nitrite solution;Anti-Rust;Diazotizing salts;Nitrite standard for aqueous matrices ready foruse
CAS NO.:7632-00-0
Empirical Formula: NaNO2
Molecular Weight: 69
MDL number: MFCD00011118
EINECS: 231-555-9
PRODUCT Properties
| Melting point: | 271 °C (lit.) |
| Boiling point: | 320 °C |
| Density | 2.17g/cm3 |
| bulk density | 1200kg/m3 |
| vapor pressure | <0.0001 hPa ( 25 °C) |
| refractive index | 1.65 |
| storage temp. | 2-8°C |
| solubility | aqueous acid: 1 - 2μl acetic acid per ml H2Osoluble |
| form | powder |
| color | White or colorless |
| Specific Gravity | 2.168 |
| Odor | Odorless |
| PH Range | 9 |
| PH | 9 (100g/l, H2O, 20℃) |
| Water Solubility | 820 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8648 |
| Stability: | Stable. Incompatible with reducing agents, strong oxidizing agents, organics and other flammable materials, finely powdered metals. Contact with combustible material may lead to fire. Hygroscopic. |
| Cosmetics Ingredients Functions | ANTICORROSIVE |
| InChIKey | LPXPTNMVRIOKMN-UHFFFAOYSA-M |
| CAS DataBase Reference | 7632-00-0(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium nitrite (7632-00-0) |
Description and Uses
Sodium nitrite is similar in name and use to sodium nitrate. Both are preservatives used in processed meats, such as salami, hot dogs, and bacon. Sodium nitrite has been synthesized by several chemical reactions that involve the reduction of sodium nitrate. Industrial production of sodium nitrite is primarily by the absorption of nitrogen oxides into aqueous sodium carbonate or sodium hydroxide. Over the years, sodium nitrite has raised some concerns about its safety in foods, but it remains in use and there are indications that it may actually be healthy. Sodium nitrite was developed during the 1960s. In 1977, the US Department of Agriculture (USDA) considered banning it but the USDA’s final ruling on the additive came out in 1984, allowing its use. Studies in the 1990s indicated some adverse effects of sodium nitrite, for instance the potential to cause childhood leukemia and brain cancers. In the late 1990s, the National Toxicity Program (NTP) began a review of sodium nitrite and proposed listing sodium nitrite as a developmental and reproductive toxicant, but a report in 2000 by NTP proposed that sodium nitrite is not a toxic substance and removed it from the list of developmental and reproductive toxicants. It is now believed that it can help with organ transplants and leg vascular problems, while preventing heart attacks and sickle cell disease.
Sodium nitrite is used to fix the colors in preserved fish and meats. It is also important(along with sodium chloride) in controlling the bacterium Clostridium botulinum, which causes botulism. Lunch meats, hams, sausages, hot dogs, and bacon are usually preserved this way.
In medicines, it is a vasodilator, intestinal relaxant, bronchodilator, and an antidote to cyanide and hydrogen sulfide poisoning.
Sodium nitrite is produced in the human body by the action of saliva on sodium nitrate, and is important in controlling bacteria in the stomach, to prevent gastroenteritis. The body produces more sodium nitrite than is consumed in food.
Sodium nitrite can react with proteins in the stomach or during cooking, especially in high heat (such as frying bacon), to form carcinogenic N-nitrosamines.To prevent this, ascorbic acid or erythorbic acid is commonly added to cured meats.
manufacture of diazo dyes, nitroso Compounds, and in many other processes of manufacture of organic chemicals; dyeing and printing textile fabrics; bleaching flax, silk, and linen.
Safety
| Symbol(GHS) |    GHS03,GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H272-H301-H319-H400 |
| Precautionary statements | P210-P220-P264-P273-P301+P310-P305+P351+P338 |
| Hazard Codes | O,T,N,Xn |
| Risk Statements | 8-25-50-22-36/38 |
| Safety Statements | 45-61-36-26 |
| RIDADR | UN 3219 5.1/PG 3 |
| WGK Germany | 3 |
| RTECS | RA1225000 |
| F | 10 |
| Autoignition Temperature | 914 °F |
| TSCA | Yes |
| HazardClass | 5.1 |
| PackingGroup | III |
| HS Code | 28341000 |
| Hazardous Substances Data | 7632-00-0(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 180 mg/kg (Smyth) |