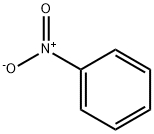
Nitrobenzene solution , Concentration: 1.00 (mg/l), analyze the standard product , 98-95-3
Synonym(s):
Nitrobenzene
CAS NO.:98-95-3
Empirical Formula: C6H5NO2
Molecular Weight: 123.11
MDL number: MFCD00007043
EINECS: 202-716-0
PRODUCT Properties
| Melting point: | 5-6 °C (lit.) |
| Boiling point: | 210-211 °C (lit.) |
| Density | 1.196 g/mL at 25 °C (lit.) |
| vapor density | 4.2 (vs air) |
| vapor pressure | 0.15 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 190 °F |
| storage temp. | Store below +30°C. |
| solubility | 1.90g/l |
| form | Liquid |
| pka | 3.98(at 0℃) |
| color | Clear yellow |
| PH | 8.1 (1g/l, H2O, 20℃) |
| Relative polarity | 4.5 |
| explosive limit | 1.8-40%(V) |
| Water Solubility | slightly soluble |
| Merck | 14,6588 |
| BRN | 507540 |
| Henry's Law Constant | 9.86 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Exposure limits | TLV-TWA 1 ppm (~5 mg/m3) (ACGIH,
MSHA, and OSHA); IDLH 200 ppm
(NIOSH). |
| Dielectric constant | 35.7(20℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong reducing agents, strong bases. Flammable. Note wide explosion limits. |
| LogP | 1.86 at 24.5℃ and pH7.9 |
| Surface tension | 43.9mN/m at 20°C |
| CAS DataBase Reference | 98-95-3(CAS DataBase Reference) |
| IARC | 2B (Vol. 65) 1996 |
| NIST Chemistry Reference | Benzene, nitro-(98-95-3) |
| EPA Substance Registry System | Nitrobenzene (98-95-3) |
Description and Uses
Nitrobenzene is a greenish-yellow crystal or yellow oily liquid, and is slightly soluble in water. The primary hazard of nitrobenzene is toxicity; however, it is also combustible. The boiling point is about 410°F, the flash point is 190°F, and the ignition temperature is 900°F. The specific gravity is 1.2, which is heavier than water, and the material will sink to the bottom. The vapor density is 4.3, which is heavier than air. Nitrobenzene is toxic by ingestion, inhalation, and skin absorption, with a TLV of 1 ppm in air. The four-digit UN identification number is 1652. The NFPA 704 designation is health 3, flammability 2, and reactivity 1. Nitrobenzene is a nitro hydrocarbon derivative, but it is not very explosive. The primary uses are as a solvent, an ingredient of metal polishes and shoe polishes, and in the manufacture of aniline.
Most nitrobenzene (97%) is used in the manufacture of aniline (IARC 1996, HSDB 2009). Miscellaneous uses include the manufacture of benzidine, quinoline, azobenzene, pyroxylin compounds, isocyanates, pesticides, rubber chemicals, pharmaceuticals, and dyes such as nigrosines and magenta. Nitrobenzene is found in soaps and shoe and metal polishes and is used as a solvent for cellulose ester, in modifying esterification of cellulose acetate, and in refining lubricating oils (HSDB 2009). Nitrobenzene also is used as a solvent in petroleum refining and the synthesis of other organic compounds, such as acetaminophen (ATSDR 1990).
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301+H311+H331-H351-H360F-H372-H412 |
| Precautionary statements | P202-P273-P280-P301+P310-P302+P352+P312-P304+P340+P311 |
| Hazard Codes | T,N,F,Xn |
| Risk Statements | 23/24/25-40-48/23/24-51/53-62-39/23/24/25-11-36/37/38-60-52/53-48/23/24/25-36-20/21/22 |
| Safety Statements | 28-36/37-45-61-28A-16-7-27-53-26 |
| OEB | B |
| OEL | TWA: 1 ppm (5 mg/m3) [skin] |
| RIDADR | UN 1662 6.1/PG 2 |
| WGK Germany | 2 |
| RTECS | DA6475000 |
| Autoignition Temperature | 899 °F |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | II |
| HS Code | 29042010 |
| Hazardous Substances Data | 98-95-3(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 600 mg/kg (PB91-108398) |
| IDLA | 200 ppm |